Cinacalcet hydrochloride preparation method
A technology of cinacalcet hydrochloride and cinnamic acid, which is applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., can solve the problems of troublesome processing, high processing cost, low yield, etc., and achieves alleviation of post-processing high pressure, low product impurity content, and the effect of simplifying operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
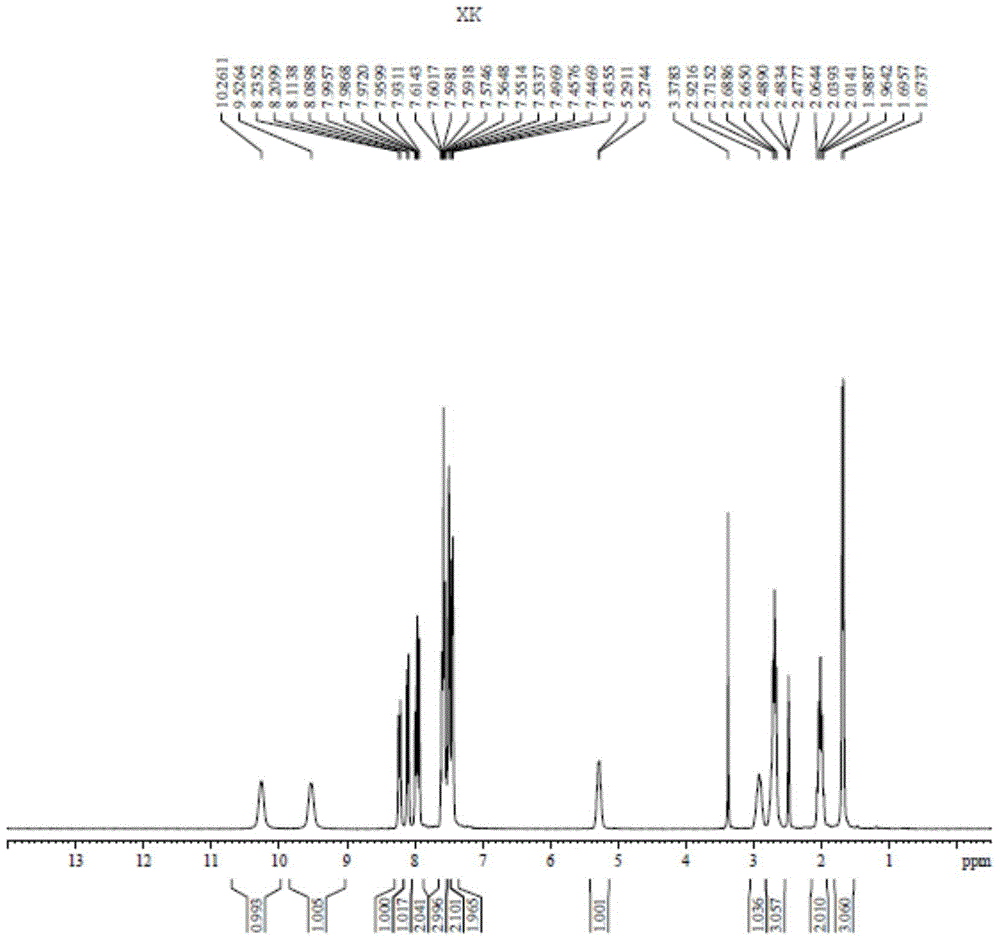
[0048] The preparation method of cinacalcet hydrochloride is as follows:
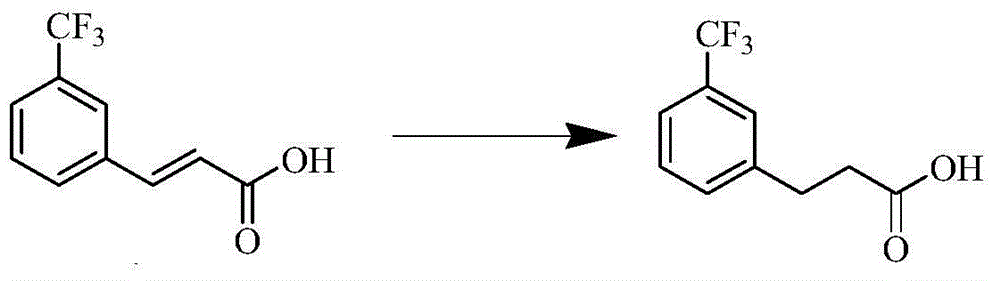
[0049] (A) Using 25 kg of trifluoromethyl cinnamic acid as a raw material, 2.5 kg of palladium carbon as a catalyst, and 150 L of methanol as a solvent, carry out hydrogenation reduction at a temperature of 0-60 ° C for 1-24 h to obtain a yellow oil Fluoromethylbenzoic acid, yield 98.0%;
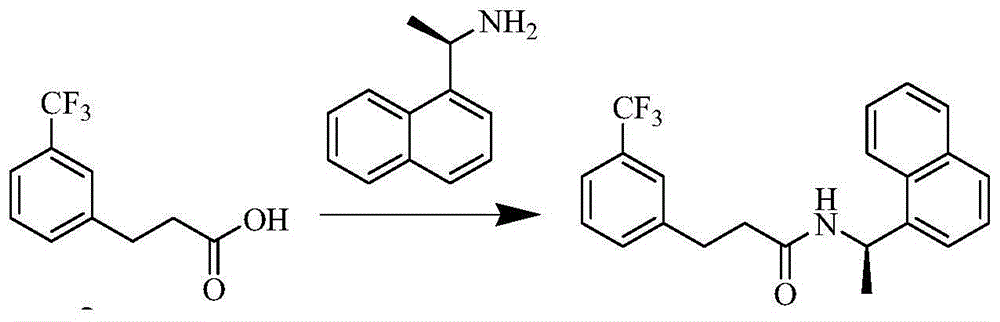
[0050] (B) Condensation reaction of m-trifluoromethylbenzoic acid and R-(1)naphthylethylamine at a mass ratio of 1:4 at a temperature of 50-200°C for 1-20h to obtain an off-white solid (R )-N-(1-(naphthalene-1-yl)ethyl)-3-(trifluoromethyl)phenyl)propionamide, the yield was 92.1%;
[0051] (C) (R)-N-(1-(naphthalene-1-yl)ethyl)-3-(trifluoromethyl)phenyl)propanamide and acetic acid in a mass ratio of 10:1 at a temperature of 0- Cinacalcet was obtained as a yellow oil after being reduced by the reducing agent boron trifluoride at 10°C, the solvent used was tetrahydrofuran, and the yield was 98.2%;
[0052] (D) reacting...
Embodiment 2
[0054] The preparation method of cinacalcet hydrochloride is as follows:
[0055] (A) Add 50kg of trifluoromethyl cinnamic acid, 0.5kg of palladium carbon and 150L of N,N-dimethylformamide in the autoclave, first replace the nitrogen with nitrogen twice, then replace the nitrogen twice with hydrogen, pass Add hydrogen to keep the pressure at 0.2-0.3MPa, carry out hydrogenation and reduction at a temperature of 30°C for 4 hours, stop stirring, evacuate, discharge and filter, wash with 20L of methanol, and concentrate the filtrate to dryness under vacuum at 40°C. Obtain 49.6 kg of yellow oil m-trifluoromethylbenzoic acid, yield 98.0%;
[0056] (B) Condensate 45kg m-trifluoromethylbenzoic acid and 14kg R-(1)naphthylethylamine at a temperature of 140°C for 5h, stop heating and cool to 40-60°C and add 100L ethyl acetate , 30L of water, separated after stirring, the water phase was extracted once with 40L of ethyl acetate, the organic phase was combined, and the organic phase was w...
Embodiment 3
[0061] The preparation method of cinacalcet hydrochloride is as follows:
[0062] (A) Add 50kg of trifluoromethylcinnamic acid, 0.25kg of palladium carbon and ethanol and N,N-dimethylformamide mixture 150L in the autoclave, wherein the mass percentage of palladium in the catalyst is 5 -10%, first replace nitrogen twice with hydrogen, then replace nitrogen twice with hydrogen, feed hydrogen to maintain the pressure of 0.2-0.3MPa, carry out hydrogenation and reduction at a temperature of 40°C for 10h, stop stirring, empty and discharge Filter, wash with 20L of methanol, and concentrate the filtrate to dryness at 40°C to obtain 48.6kg of m-trifluoromethylbenzoic acid as a yellow oil, with a yield of 98.0%;
[0063] (B) Condensate 45kg m-trifluoromethylbenzoic acid and 36kg R-(1)naphthylethylamine at a temperature of 150°C for 6h, stop heating and cool to 40-60°C and add 100L ethyl acetate , 30L of water, separated after stirring, the water phase was extracted once with 40L of et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com