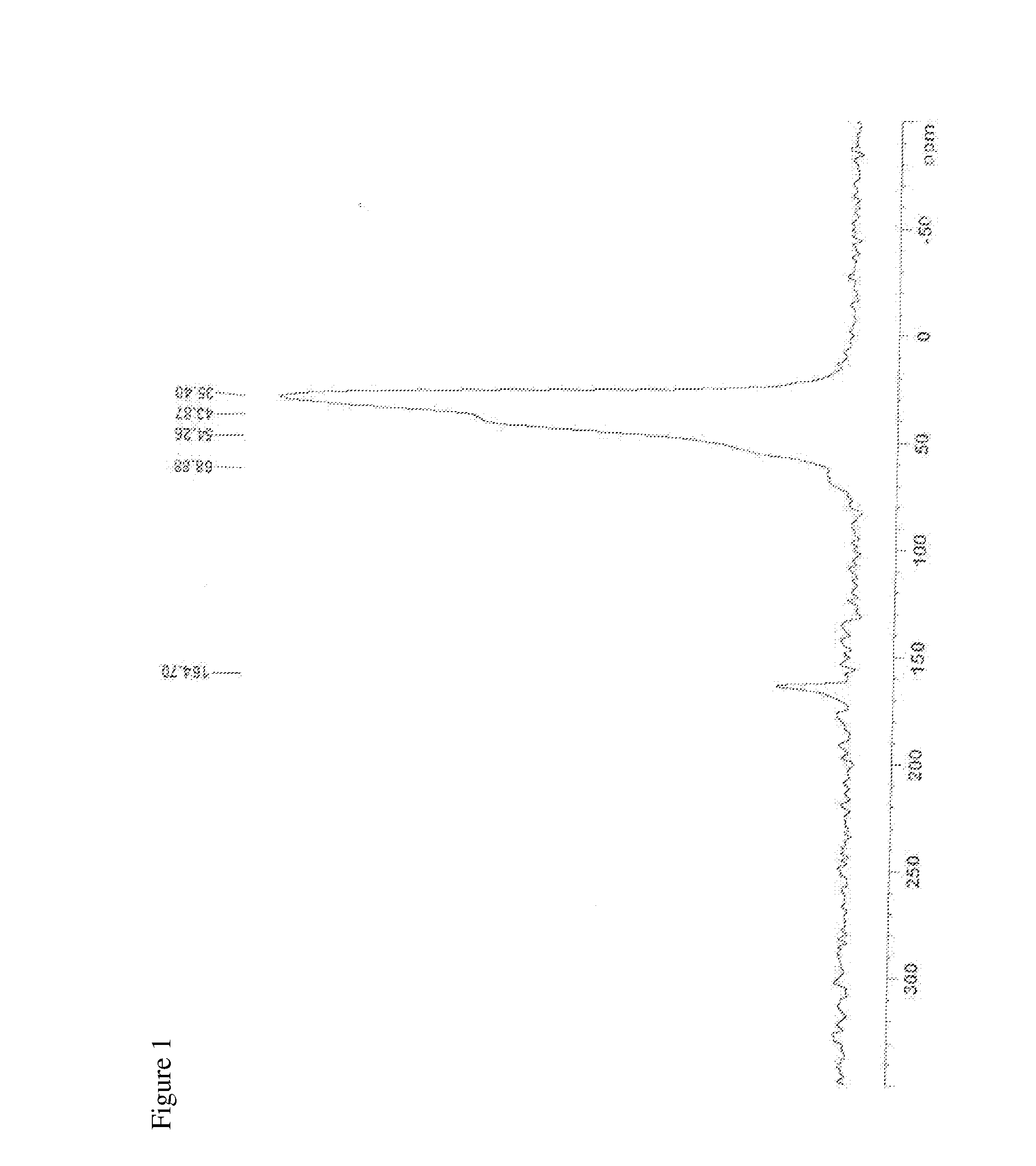
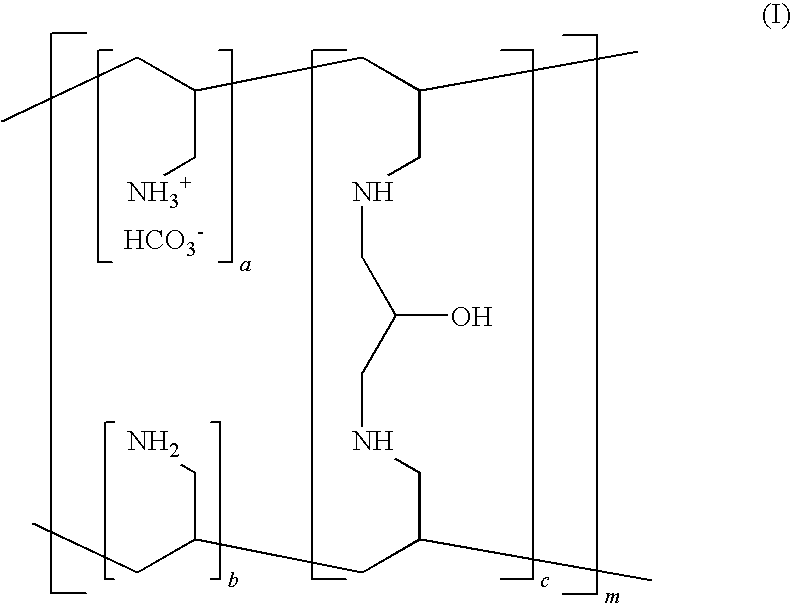
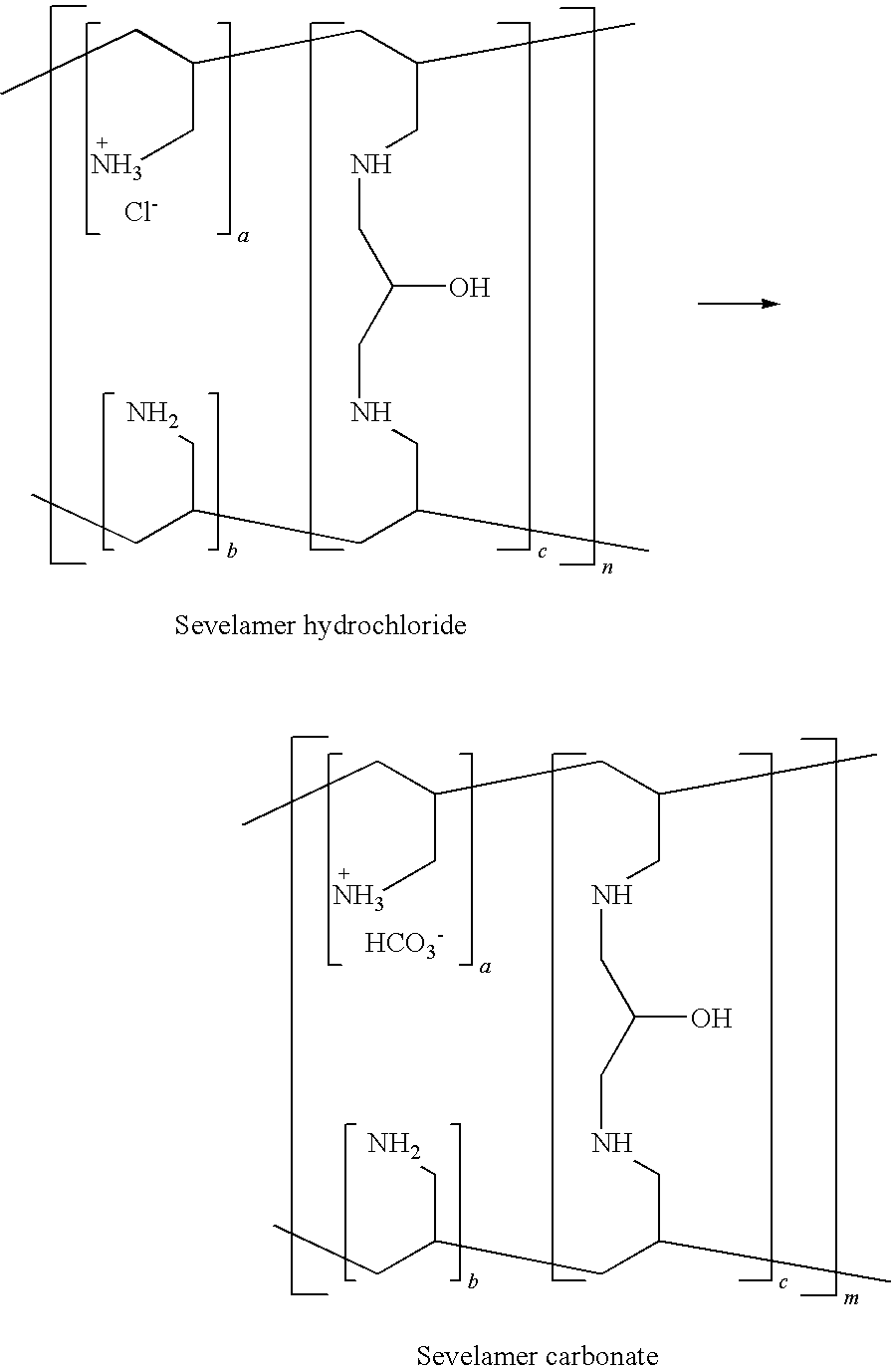
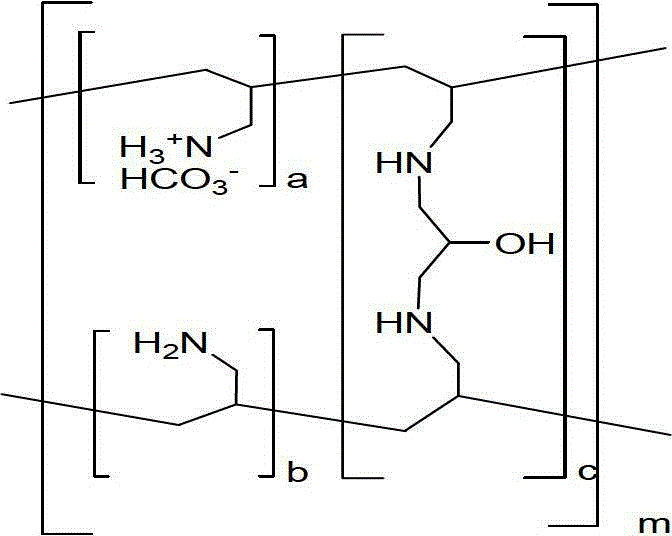
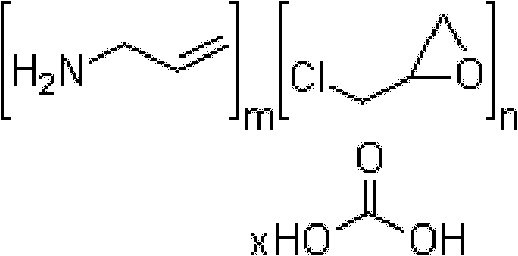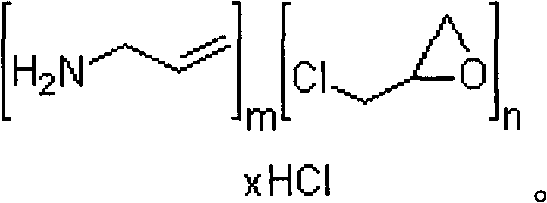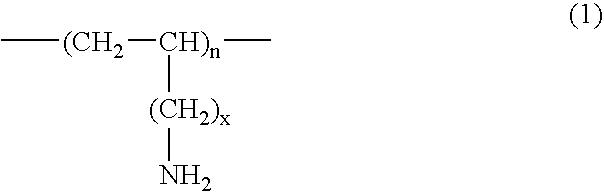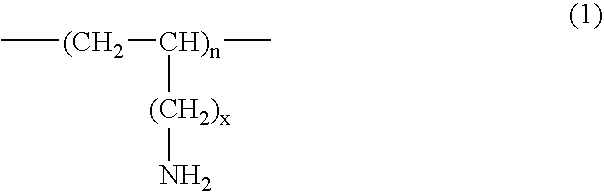Patents
Literature
77 results about "Sevelamer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
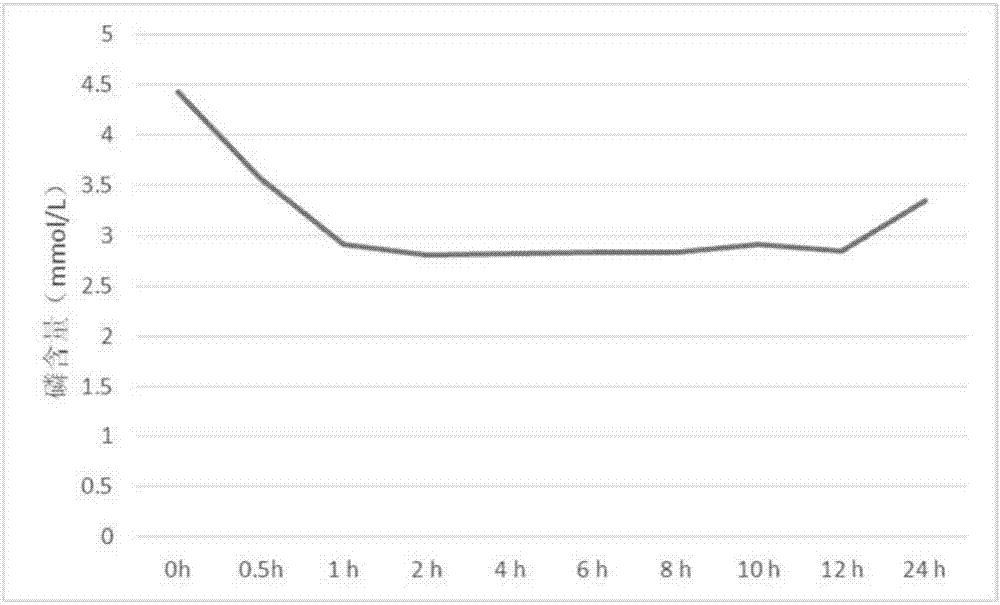
Sevelamer is used to lower high blood phosphorus (phosphate) levels in patients who are on dialysis due to severe kidney disease.
Pharmaceutical compositions comprising phosphate binder, calcium receptor-active compound and/or active vitamin d
InactiveUS20130085121A1Maintain good propertiesImprove bioavailabilityOrganic active ingredientsBiocideMineral bone diseaseCalcium Binder
The present invention is an oral solid pharmaceutical compositions for the treatment of kidney diseases and mineral bone disorder including a phosphate binder, a calcium receptor-active compound and at least one pharmaceutically acceptable excipient, the invention further including a method for preparing the pharmaceutical compositions including the steps of granulating cinacalcet and / or sevelamer and / or vitamin D by one of a wet and a dry granulation process, each with at least one pharmaceutically acceptable excipient to form cinacalcet granules and / or sevelamer granules and / or vitamin D granules, mixing at least two of the cinacalcet granules, sevelamer granules and vitamin D granules to form a granules mixture and compressing the granules mixture to tablets or encapsulating the granules mixture into capsules or pulverizing the granules mixture into a dispersion powder.
Owner:WEIFANG SYNERPHARM
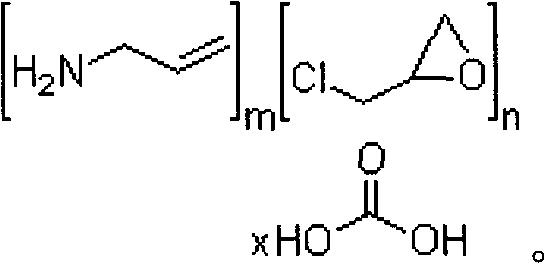
Pharmaceutical Compositions Comprising Phosphate-Binding Polymer
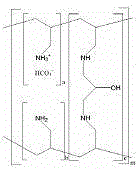
The present invention discloses pharmaceutical composition comprising phosphate binding polymers such as Sevelamer carbonate substantially free of monovalent anion other than bicarbonate anion. Particularly, monovalent anion content is less than about 0.05% (w / w). Disclosed are compositions comprising wet granulated Sevelamer carbonate free of added metal ions and / or added monovalent anion source.
Owner:USV LTD
Process for Preparation of Sevelamer Carbonate
InactiveUS20100331516A1Simple processSave energyOrganic active ingredientsSevelamerMedicinal chemistry
Owner:USV LTD
Sevelamer carbonate medical tablet composition and preparation method thereof
The invention discloses a sevelamer carbonate medical tablet composition and a preparation method thereof. The tablet composition comprises the following components in parts by weight: 60-95 parts of sevelamer carbonate, 5-30 parts of crospovidone and 0.1-10.0 parts of silicon dioxide. The preparation method of the tablet composition comprises the following steps: a. sevelamer carbonate is mixed with crospovidone; b. the mixture obtained in step a is pelletized; c. silicon dioxide is mixed with the particles produced in step b to form tablets; d. the tablets obtained is film-coated by water-soluble coating materials. The tablet composition provided by the invention is characterized in that the formability is good, the rigidity is high, the disintegration is quick, and the disintegration is less affected by the rigidity.
Owner:NANJING LIFENERGY R & D +1
Pharmaceutical Compositions Comprising Phosphate-Binding Polymer
The present invention discloses pharmaceutical composition comprising phosphate binding polymers such as Sevelamer carbonate substantially free of monovalent anion other than bicarbonate anion. Particularly, monovalent anion content is less than about 0.05% (w / w). Disclosed are compositions comprising wet granulated Sevelamer carbonate free of added metal ions and / or added monovalent anion source.
Owner:USV LTD
Pharmaceutical Compositions of Sevelamer
InactiveUS20120219626A1Urinary disorderSynthetic polymeric active ingredientsCoated tabletsLACTOSE MONOHYDRATE
The invention relates to a pharmaceutical immediate release tablet comprising a core comprising 70-85 weight percent of sevelamer carbonate, calculated as an anhydrous compound, 10-25 weight percent of lactose monohydrate and, optionally, a water soluble film coat surrounding the to a process of making such tablets, to their use in medicine, and to the use of polyvinyl alcohol-polyethylene glycol graft copolymer for making such coated tablets.
Owner:SYNTHON BV
Pharmaceutical compositions comprising phosphate-binding polymer
The present invention discloses pharmaceutical composition comprising phosphate binding polymers such as Sevelamer carbonate substantially free of monovalent anion other than bicarbonate anion. Particularly, monovalent anion content is less than about 0.05% (w / w). Disclosed are compositions comprising wet granulated Sevelamer carbonate free of added metal ions and / or added monovalent anion source.
Owner:USV LTD
Underwater-dispersible tablet of Sevelamer carbonate
ActiveCN102641251ANo suspension stabilityNo special tasteOrganic active ingredientsPill deliveryAdjuvantMedicine
The invention relates to an underwater-dispersible tablet of Sevelamer carbonate, which is characterized by containing Sevelamer carbonate salt and a low-melting-point water-soluble adjuvant and is applicable to the field of medical technologies.
Owner:TIANJIN PACIFIC PHARMA +1
Manufacturing Process Of Making Polymeric Amine Salts
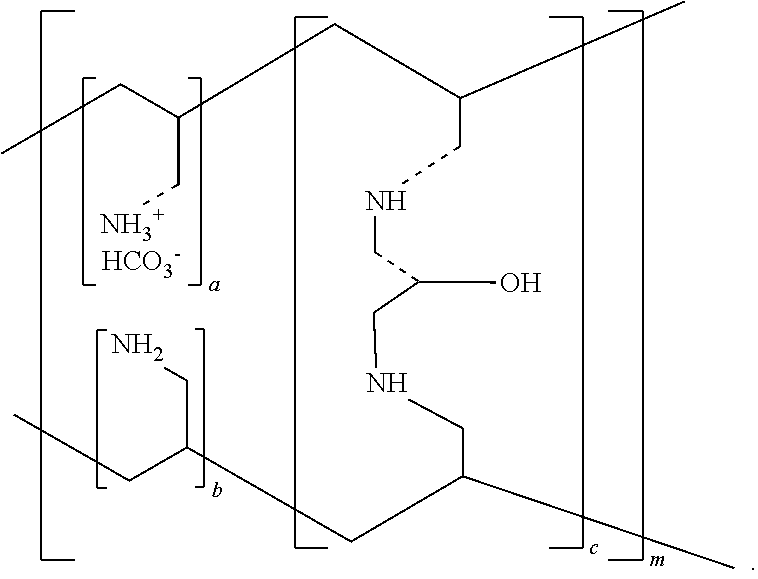
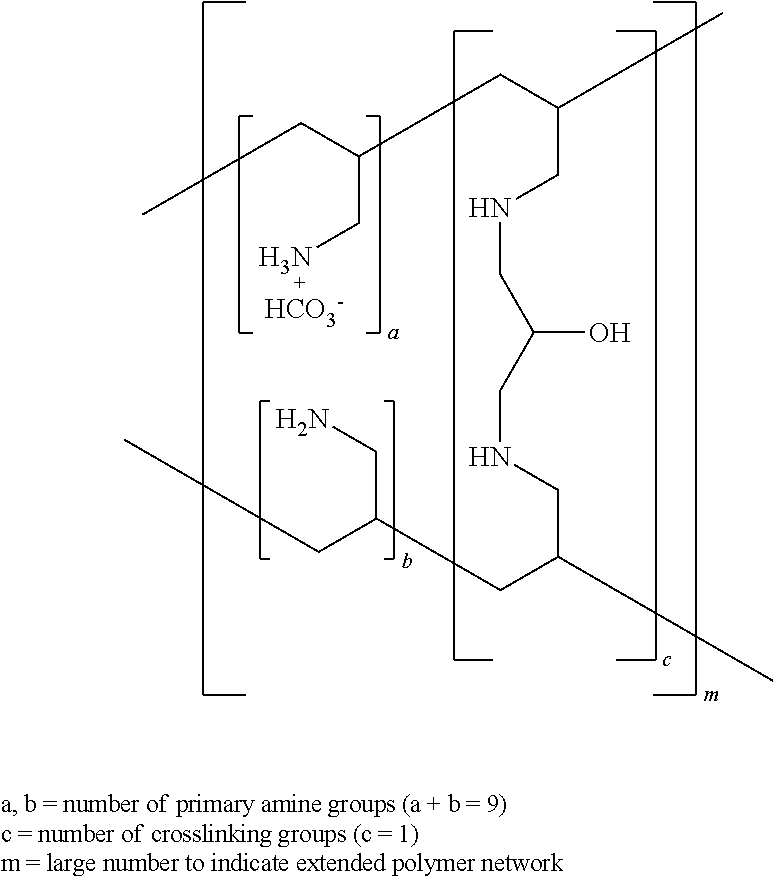
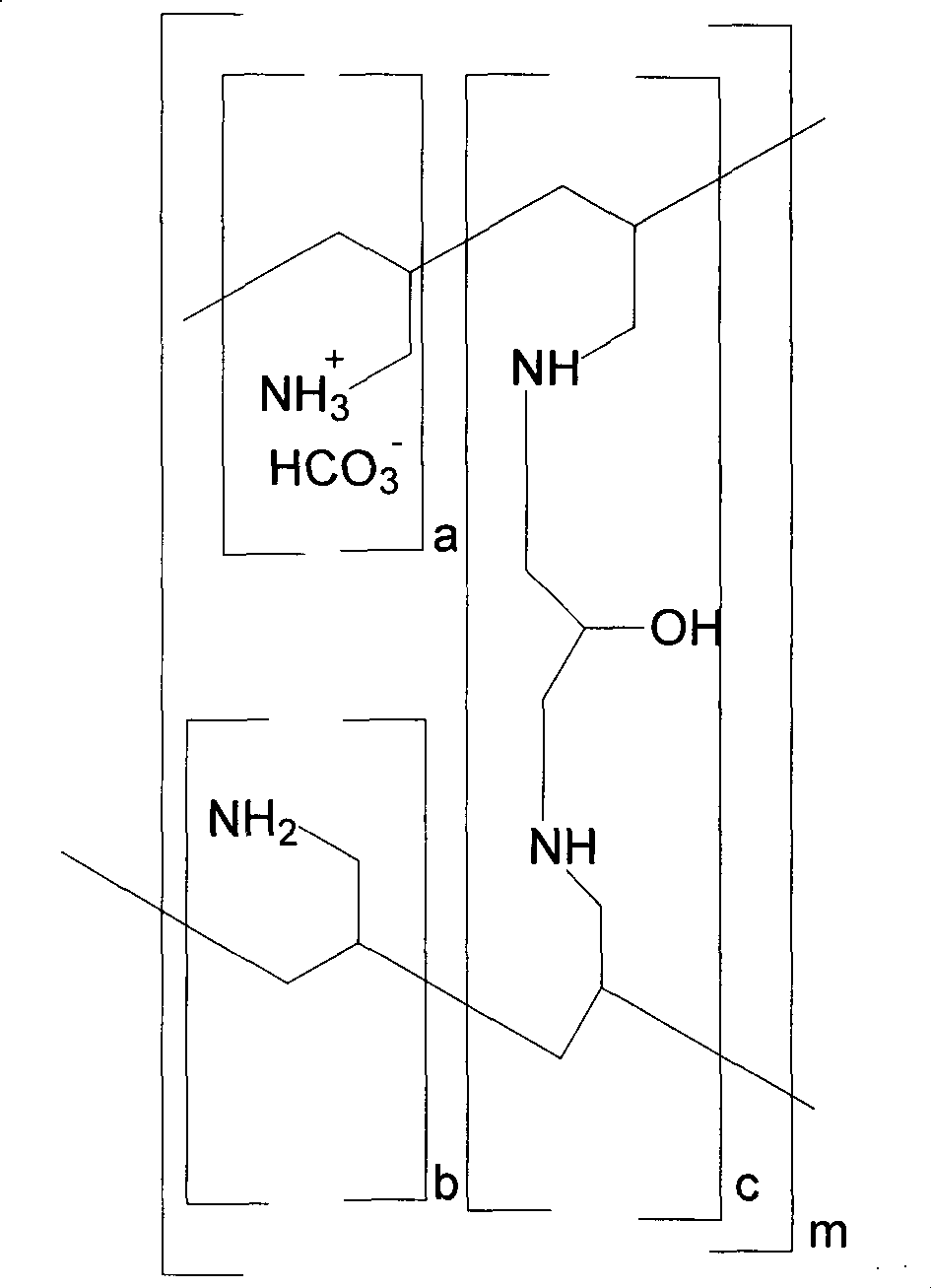
ActiveUS20100137542A1Control reaction rateSmall particle sizePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsCross-linkChloride salt
A novel process of manufacturing sevelamer carbonate from a polyallylamine carbonate or bicarbonate chloride salt. Process for manufacture of carbonate and / or bicarbonate salts of water insoluble polymers containing amino groups that are useful as anion binders in the gastrointestinal (GI) system. The process arranges the polyallylamine chain in a solution in such a way that the cross-linking reaction with epichlorohydrin can be controlled at a desired reaction rate.
Owner:NAVINTA
Sevelamer carbonate effervescent tablets and preparation method thereof
InactiveCN104434866AHigh hardnessImprove medication complianceOrganic active ingredientsMetabolism disorderEffervescent tabletUse medication
Owner:JINAN KANGHE MEDICAL TECH
Cross-linked polyallylamine tablet core
InactiveUS20100330175A1Reduce cholesterolOrganic active ingredientsMetabolism disorderCross-linkCholesterol
A method and a composition for making a composition, tablet, or tablet core having cross-linked polyallylamine salts such as sevelamer hydrochloride, sevelamer carbonate, or colesevelam hydrochloride, that may be used for treating hyperphosphatemia or reducing cholesterol. The method involves blending of a cross-linked polyallylamine salt with a water soluble excipient, optionally with water, an additive and / or a lubricant, and further tableting the resulting blend to form tablets and tablet cores.
Owner:NAVINTA
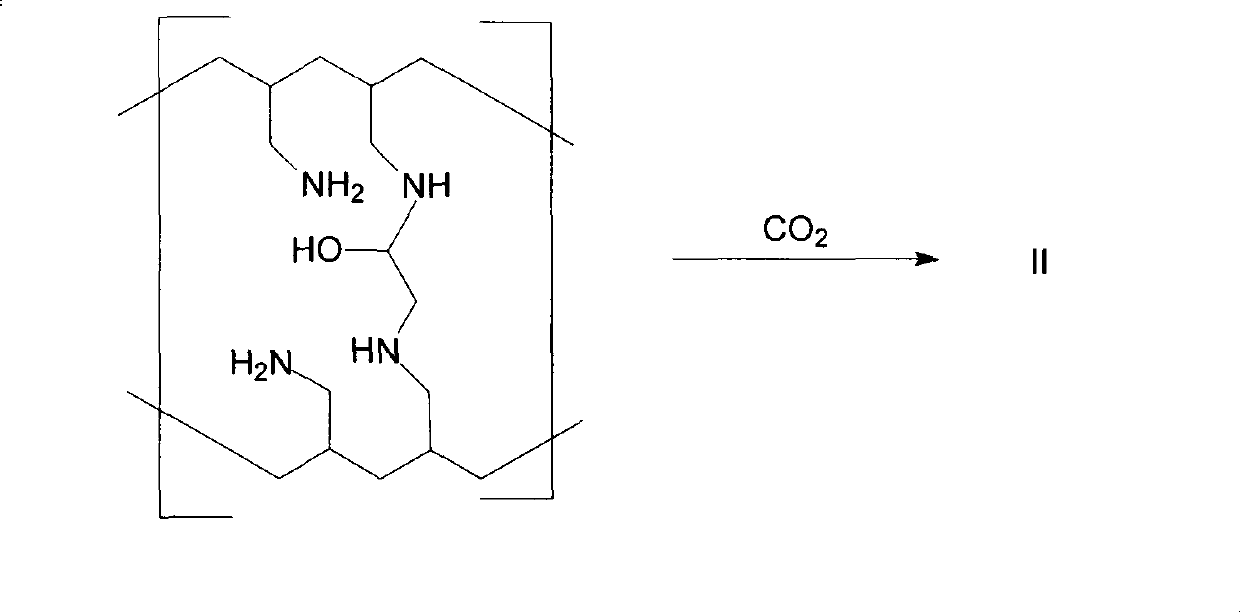
Method for preparing sevelamer carbonate
The invention provides a method for preparing sevelamer carbonate. The method comprises the following steps: a) polymerizing poly(allylamine hydrochloride) and 1, 3-dichloro-2-propanol at the temperature of between 30 and 70DEG C and the pH of 7-10, washing, and collecting the polymerized product to obtain sevelamer hydrochloride; b) dispersing the sevelamer hydrochloride in an alkali solution at the pH of 8-12 to obtain alkaline sevelamer; c) washing the alkaline sevelamer with water until neutrality to obtain an alkaline sevelamer cationic resin; and d) mixing a carbonic acid solution and the alkaline sevelamer cationic resin to obtain the sevelamer carbonate. By the method for preparing the sevelamer carbonate, the sevelamer carbonate has high purity; and in addition, by a Soxhlet extraction method, the mass transfer velocity can be improved and the reaction time is saved.
Owner:NEW FOUNDER HLDG DEV LLC +2
Preparation for quickly disintegrating fatty amine polymer salt
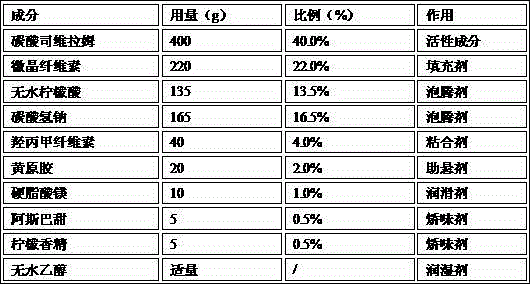
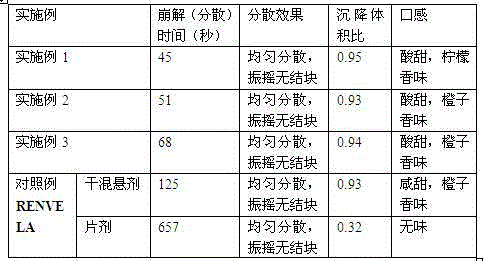
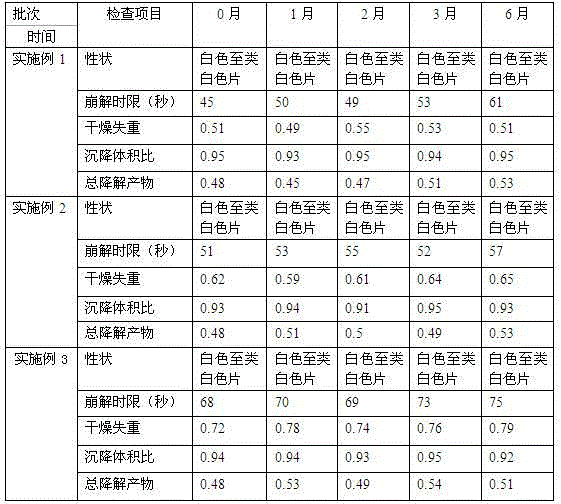
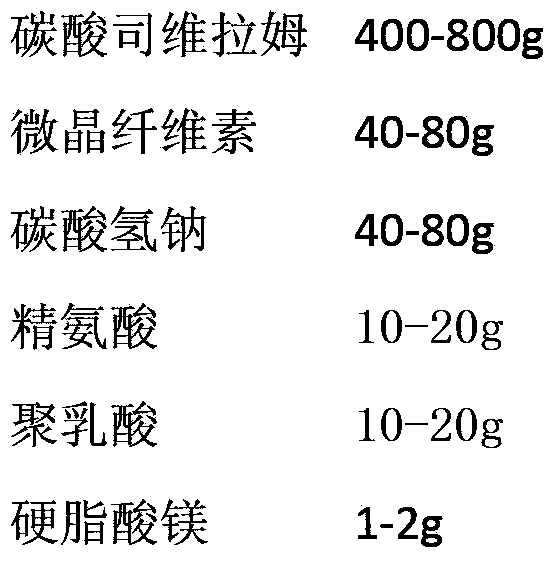
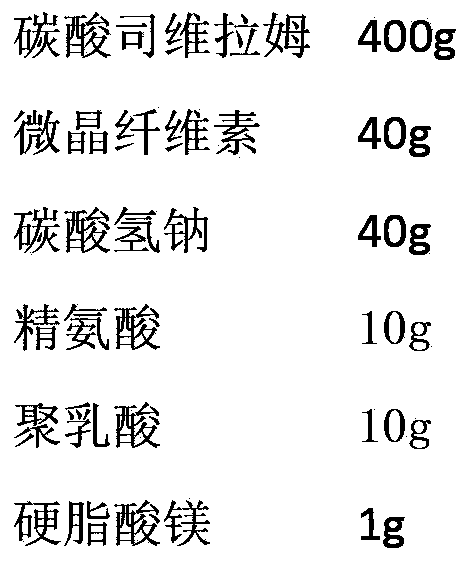
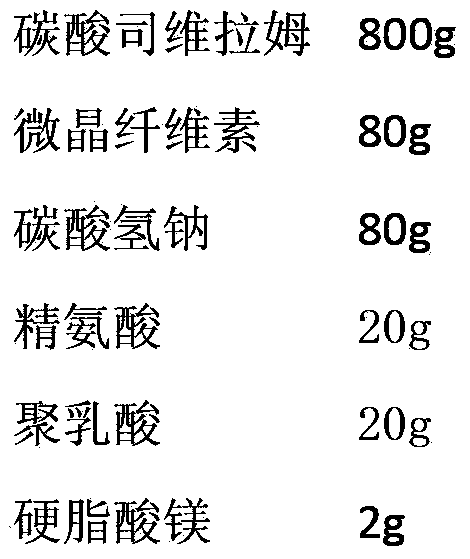
ActiveCN103393610AOral effect is goodImprove bindingOrganic active ingredientsMetabolism disorderSodium bicarbonateArginine
The invention relates to a preparation for quickly disintegrating fatty amine polymer salt, and particularly relates to a sevelamer carbonate tablet. The formula of the preparation comprises the following components: 400-800g of sevelamer carbonate, 40-80g of microcrystalline cellulose, 40-80g of sodium hydrogen carbonate, 10-20g of arginine, 10-20g of polylactic acid and 1-2g of magnesium stearate. The preparation method comprises the following steps: preparing the active component (sevelamer carbonate) and the auxiliary components for later use; uniformly mixing the sevelamer carbonate, the microcrystalline cellulose, the sodium hydrogen carbonate, the arginine and the polylactic acid; adding water, and uniformly mixing; granulating through a dry method; then adding the magnesium stearate, and uniformly mixing; and tabletting.
Owner:CHANGZHOU FANGYUAN PHARMA
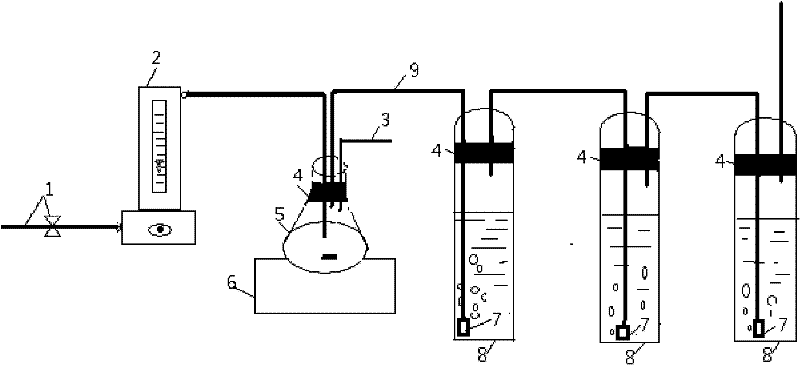
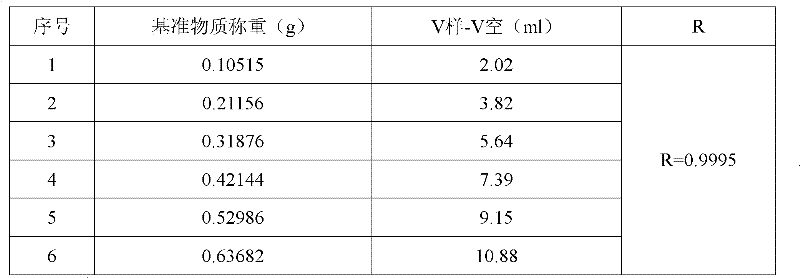
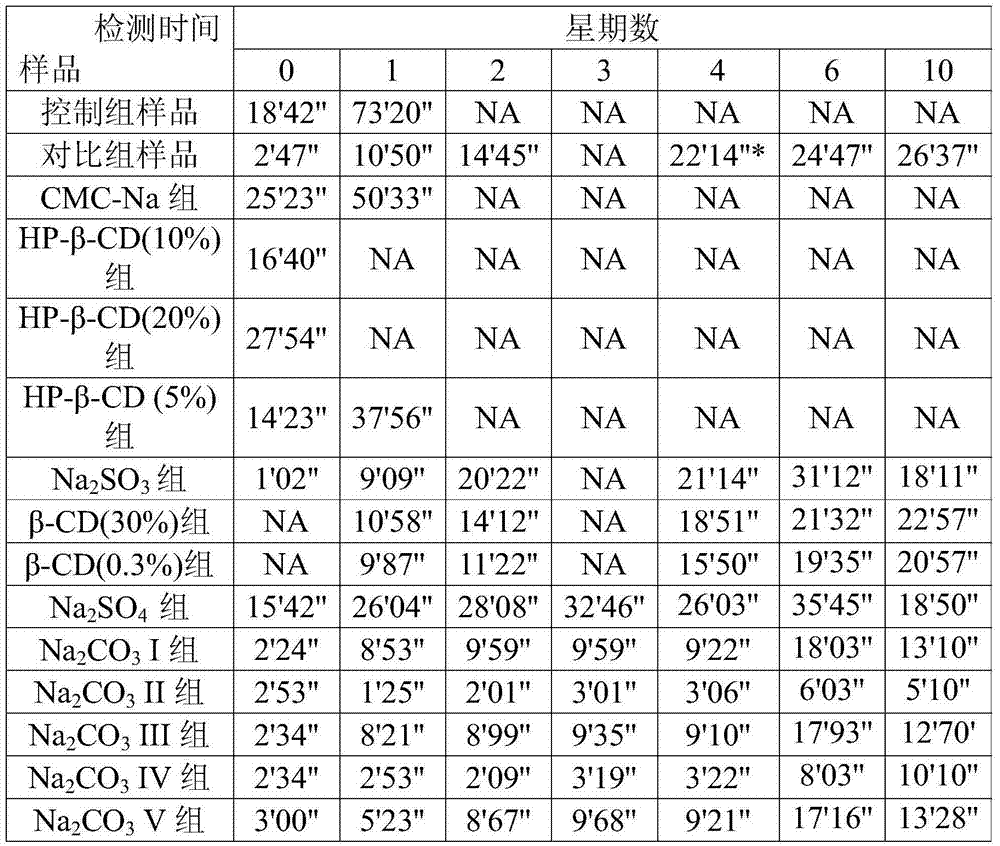
Method for measuring carbonic acid content in Sevelamer carbonate
InactiveCN102680309AMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationRelative standard deviationOperability
The invention discloses a method for measuring carbonic acid content in Sevelamer carbonate. The method includes: converting carbonic acid in Sevelamer carbonate to carbon dioxide in the condition of heating and / or adding excessive acid, conveying carbon dioxide into alkaline solution by aid of carrier gas, and measuring absorptive amount of carbon dioxide in alkaline solution to obtain carbonic acid content in Sevelamer carbonate. The method can effectively control the quality of Sevelamer carbonate and has the advantages of being clear in method principle, strong in specificity, high in accuracy, good in precision degree, low in cost, strong in operability and the like, and in addition, inspection devices are simple and can be obtained easily.
Owner:NANJING LIFENERGY R & D
Sevelamer carbonate dry suspension agent and preparation method thereof
InactiveCN104800165AEvenly distributedImprove stabilityOrganic active ingredientsPowder deliverySuspending AgentsBioavailability
The invention provides a sevelamer carbonate dry suspension agent and a preparation method thereof. The sevelamer carbonate dry suspension agent comprises the followings: 40 to 90 parts of sevelamer carbonate, 500 to 2000 parts of a filling agent, 50 to 180 parts of a corrigent, 40 to 100 parts of a suspending agent, and 10 to 40 parts of a flocculating agent. The sevelamer carbonate dry suspension agent provided by the invention is uniform in distribution, excellent in stability, has a large distribution area in the stomach and intestine, is quickly absorbed, and high in bioavailability, takes effects quickly, and has a drug effect superior to that of a sevelamer carbonate premixing agent.
Owner:CP PHARMA QINGDAO CO LTD
Sevelamer carbonate tablet and preparation method thereof
The invention provides a sevelamer carbonate tablet and a preparation method thereof, belonging to the technical field of medicines. The sevelamer carbonate tablet mainly comprises a diluent, a modifier, a lubricant, a flow aid, and a coating agent. The sevelamer carbonate is an unabsorbable crosslinked polymer capable of binding with phosphorus, and is used for treating hyperphosphatemia. The sevelamer carbonate tablet is safe and effective, stable in quality, low in cost, and convenient to take, and the compliance of patients is enhanced.
Owner:CP PHARMA QINGDAO CO LTD
Sevelamer carbonate tablet and preparation method thereof
ActiveCN104739786AStable disintegration timeImprove effectivenessOrganic active ingredientsMetabolism disorderSevelamerHigh effectiveness
The invention discloses a sevelamer carbonate tablet including sevelamer carbonate and a stabilizer, wherein the stabilizer is sodium carbonate, and accounts for at least 0.01% of the weight of sevelamer carbonate. The invention also discloses a preparation method of the sevelamer carbonate tablet. The method includes uniformly mixing an excipient, sevelamer carbonate, stabilizer and water, tabletting and coating to obtain the sevelamer carbonate tablet. The sevelamer carbonate tablet provided by the invention employs sodium carbonate or beta-cyclodextrin as a stabilizer, so as to ensure that the prepared sevelamer carbonate tablet has shorter disintegration time, and higher effectiveness of active ingredients to the patients.
Owner:HANGZHOU MINSHENG BINJIANG PHARMA CO LTD
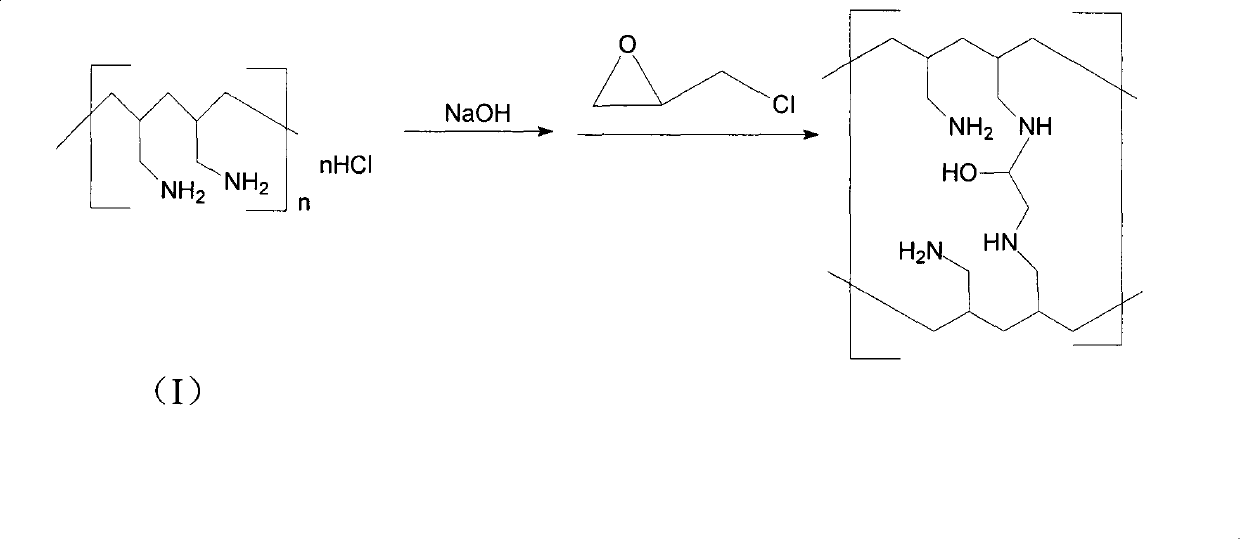
Preparation method of sevelamer carbonate
InactiveCN103864972AMeet the requirementsGood molding effectMetabolism disorderPhosphoric acidRoom temperature
The invention discloses a preparation method of sevelamer carbonate. The preparation method comprises the steps: dissolving polyallylamine hydrochloride with the average molecular weight of 8000-20000 in water, adding sodium hydroxide, then cooling to the room temperature, adding epoxy chloropropane, stirring until being viscous, and placing for 18-24 hours at the room temperature, wherein the weight part ratio of polyallylamine hydrochloride, sodium hydroxide and epoxy chloropropane is 1:0.2-0.8:0.2-0.8. Experimental results show that when the molecular weight of polyallylamine hydrochloride is in a range of 8000-20000, preferably in a range of 10000-15000, the prepared sevelamer carbonate has the advantages of good formability of tablets, high hardness and easy coating, moreover, has the disintegration time less than 3 minutes, can be fully mixed with food, reaches an effect of full complexation of phosphoric acid, and is more beneficial for patients to use.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Method for preparing sevelamer carbonate
The invention provides a method for preparing sevelamer carbonate. The method comprises the following steps: a) washing alkaline sevelamer with water until neutrality to obtain an alkaline sevelamer cationic resin; and b) mixing a carbonic acid solution and the alkaline sevelamer cationic resin to obtain the sevelamer carbonate. By the method for preparing the sevelamer carbonate, the sevelamer carbonate has high purity; and in addition, by a Soxhlet extraction method, the mass transfer velocity can be improved and the reaction time is saved.
Owner:NEW FOUNDER HLDG DEV LLC +2
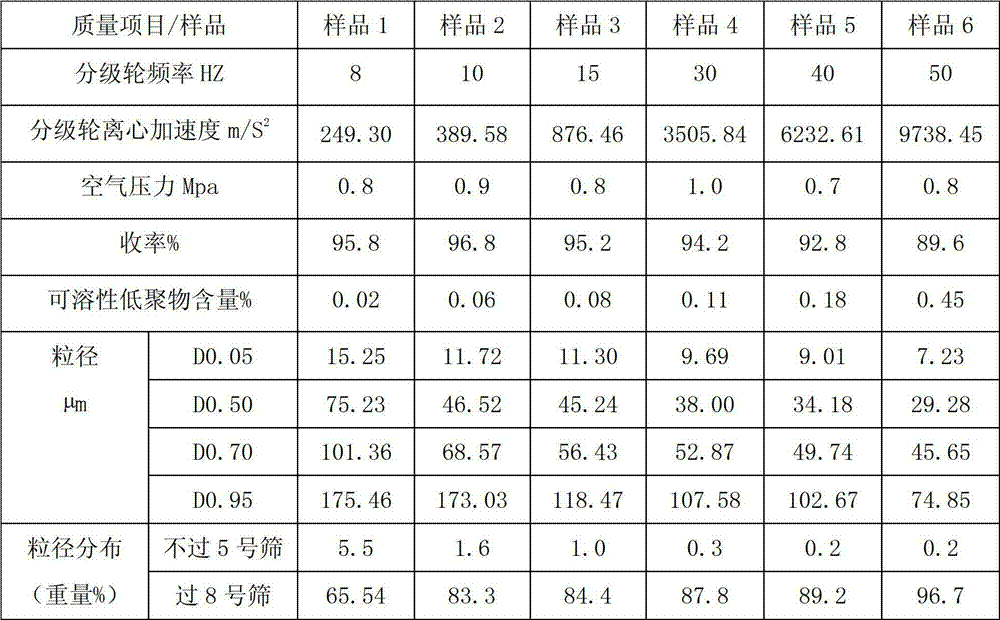
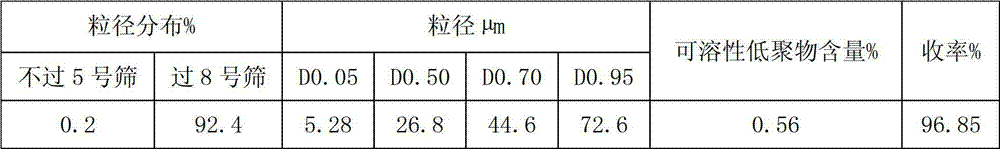
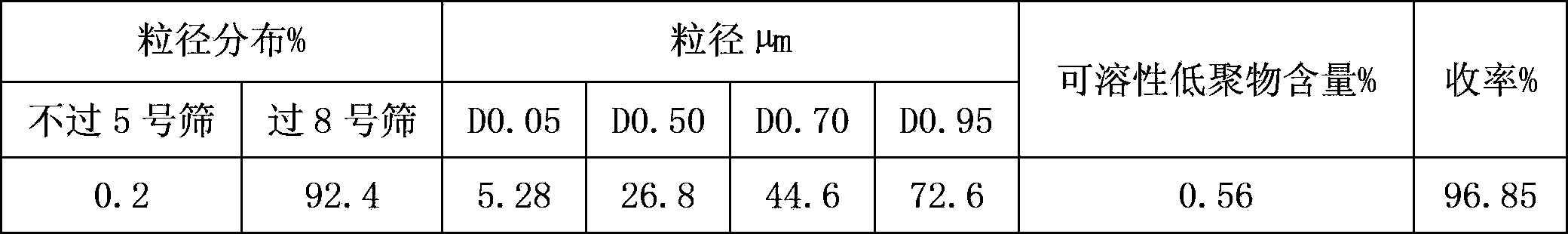
Sevelamer carbonate crude drug for preparing tablets, preparation method and application thereof
ActiveCN102895204AFlat surfaceLow friabilityOrganic active ingredientsMetabolism disorderOligomerSevelamer
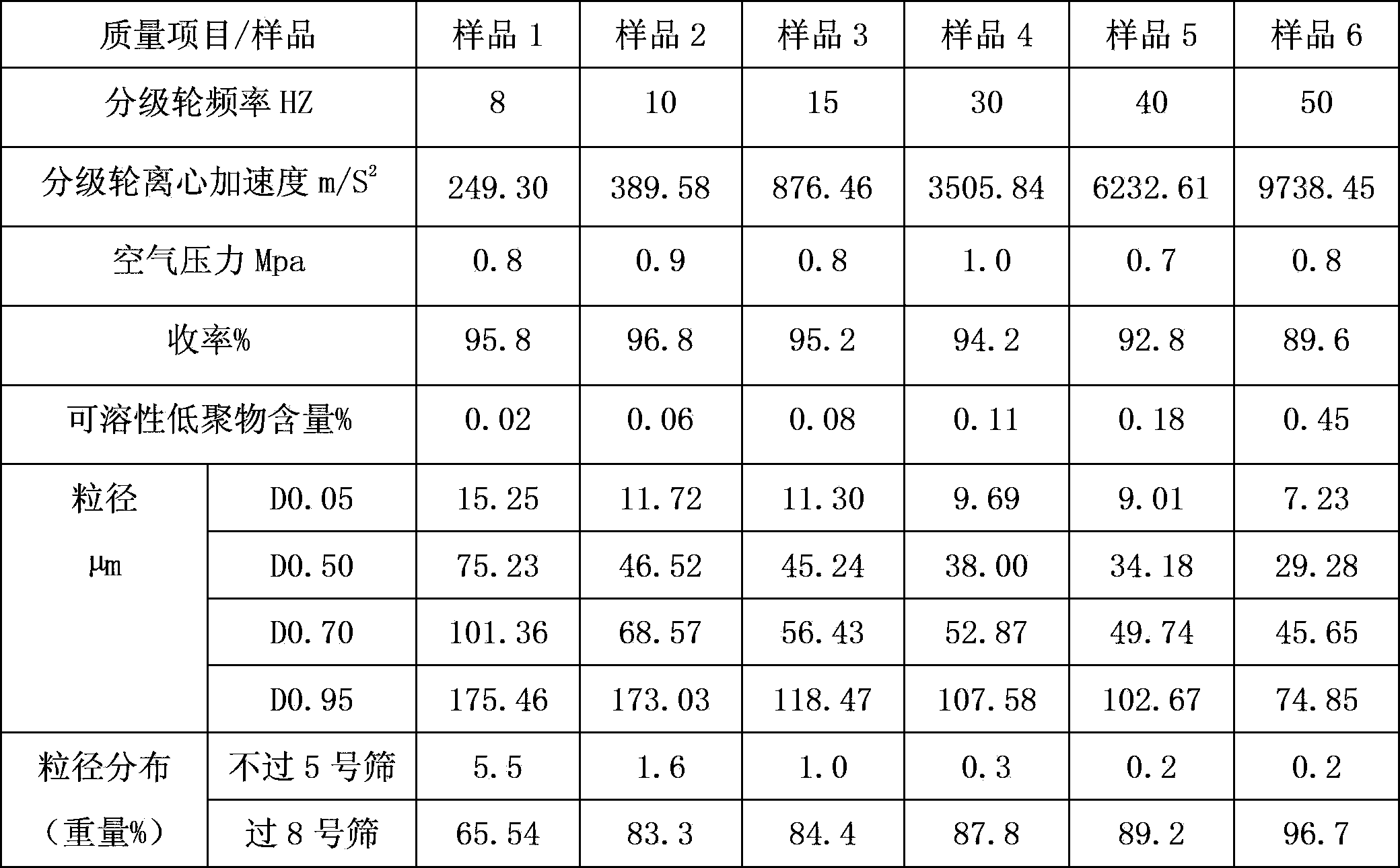
The invention belongs to the field of pharmacy, and relates to a sevelamer carbonate crude drug for preparing tablets, a preparation method and application of the sevelamer carbonate crude drug. The particle size of the sevelamer carbonate crude drug meets the conditions: D 0.95 is less than or equal to 180mu m, D 0.50 is less than or equal to 50mu m, and D 0.05 is more than or equal to 9mu m at the same time. The polyallylamine carbonate has the soluble oligomer (0.2%) within the particle size range. The tablets prepared from the sevelamer carbonate crude drug have the advantages of being smooth in surfaces, good in hardness and low in friability.
Owner:NANJING LIFENERGY R & D +1
Preparation process of sevelamer carbonate
InactiveCN105732865AAvoid wastingThe amount added is accurateOrganic active ingredientsMetabolism disorderSevelamerCarbonate
The invention relates to the technical field of production of sevelamer carbonate. A preparation process of sevelamer carbonate comprises the steps as follows: firstly, an epichlorohydrin acetonitrile solution is added to a poly (allylamine hydrochloride) solution in proportion, curing is performed, and polymerized jelly is obtained; after polymerization, the jelly is milled to 50 meshes by a colloid mill, then a 4% sodium hydroxide aqueous solution is added, the stirring reaction is performed, and sevelamer alkali is obtained; finally, purified water is added to the sevelamer alkali, then the temperature is raised, carbon dioxide is fed in, centrifugation and drying are preformed after heat-preserved stirring reaction, and sevelamer carbonate is obtained. By adopting the preparation process of sevelamer carbonate, the product energy consumption can be greatly lowered, the product quality is improved, and the problem of production of waste water is solved; meanwhile, the labor intensity can also be reduced, and the labor productivity is raised; the product quality is improved, the qualified rate of finished products reaches 99.6%, and the yield reaches 64.7%.
Owner:JIANGSU TIANHE PHARMA CO LTD
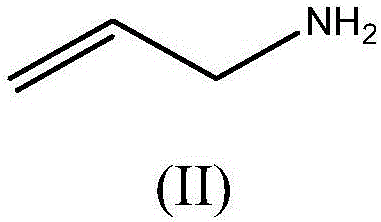
Method for preparing poly(allylamine) hydrochloride and derivatives therefrom
The present invention provides a method for preparing poly(allylamine) hydrochloride, sevelamer hydrochloride, sevelamer carbonate and colesevelam hydrochloride. The present invention also relates to a process for preparing oly(allylamine) hydrochloride, sevelamer hydrochloride, sevelamer carbonate and colesevelam hydrochloride with low allylamine content and high specific gravity.
Owner:FORMOSA LAB
Measuring method of carbonate content in sevelamer carbonate
InactiveCN106442860AFully absorbedSuitable for daily quality managementChemical analysis using titrationSevelamerBarium hydroxide
The invention relates to a measuring method of the carbonate content in sevelamer carbonate, and belongs to the technical field of medicine quantitative analysis. The method comprises the following steps: (1) installing a carbon dioxide generating device and an absorption device; (2) preparing a barium oxide absorption solution; (3) precisely weighing 1g of sevelamer carbonate, and adding the sevelamer carbonate into the carbon dioxide generating device; (4) adding the barium oxide absorption solution into the absorption device; (5) adding sulfuric acid into the sevelamer carbonate in the step (3); (6) performing titration on the barium oxide absorption solution by hydrochloric acid, and calculating the carbonate content in the sevelamer carbonate. The measuring method has the advantages that simplicity, convenience and high speed are realized; the cost is low; the accuracy and the precision are high; the measuring method is more suitable for daily quality management by a production department.
Owner:SHANDONG XINHUA PHARMA CO LTD
Process for the preparation of sevelamer
The present invention relates to a process for the preparation of sevelamer, in particular sevelamer hydrochloride and sevelamer carbonate / bicarbonate, by means of a process that allows sevelamer to be obtained with good yields and using conventional reactors, without requiring to use specific and expensive equipment.
Owner:LAB CHIM INTERNAZ
Sevelamer carbonate dry suspension for oral administration
ActiveCN107375218AImprove complianceReduce the frequency of takingOrganic active ingredientsMetabolism disorderSucroseOral medication
The invention relates to a sevelamer carbonate dry suspension. The sevelamer carbonate dry suspension comprises 35-55% of a suspending aid, 15-25% of a filler, 5-10% of a binder, 2-5% of dextrin, 2-5% of magnesium stearate, 0.1-1% of sucrose and 0.1-1% of titanium dioxide. The sevelamer carbonate dry suspension can improve action time of sevelamer carbonate in the human body and improve drug compliance.
Owner:CP PHARMA QINGDAO CO LTD
Method for lowering serum glucose
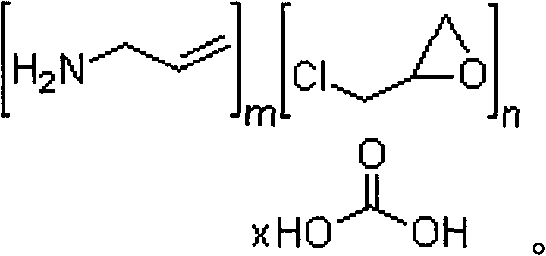
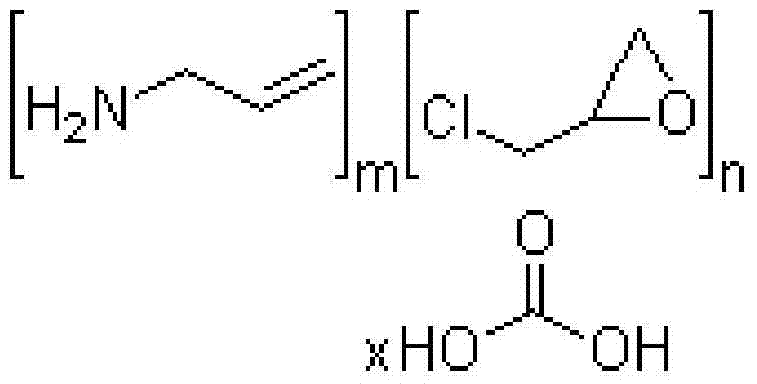
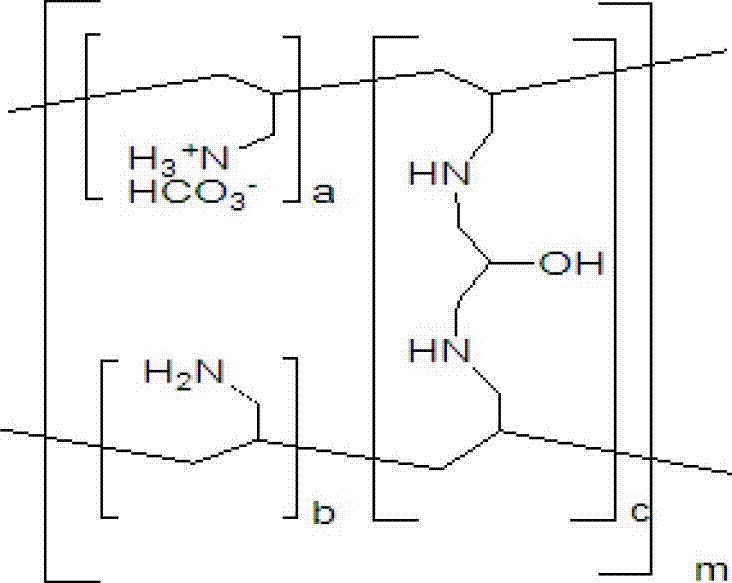
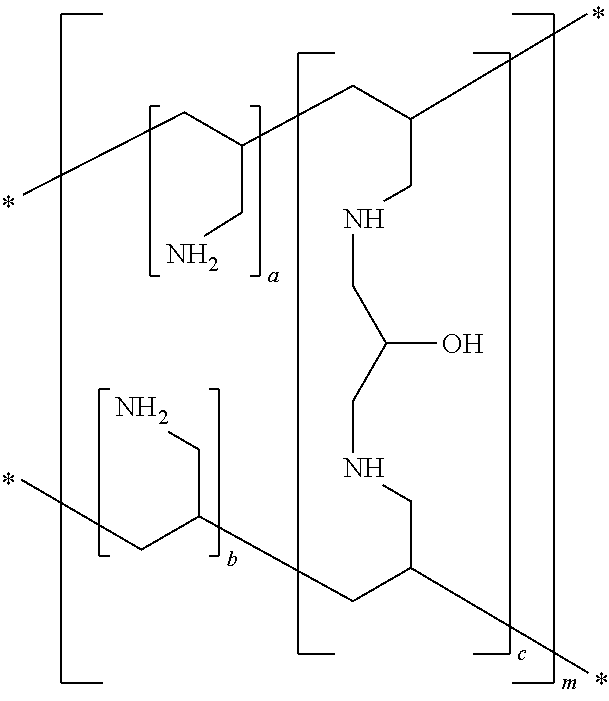
InactiveUS20070286841A1Avoids and minimizes absorptionMetabolism disorderSynthetic polymeric active ingredientsAcute hyperglycaemiaCross-link
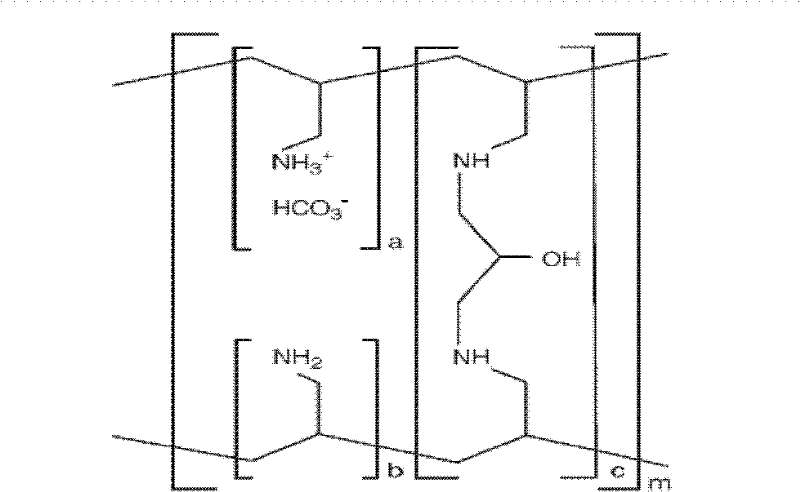
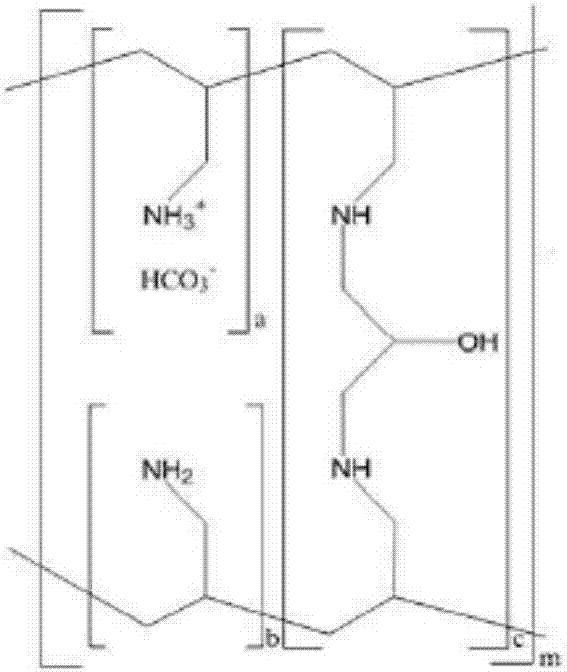
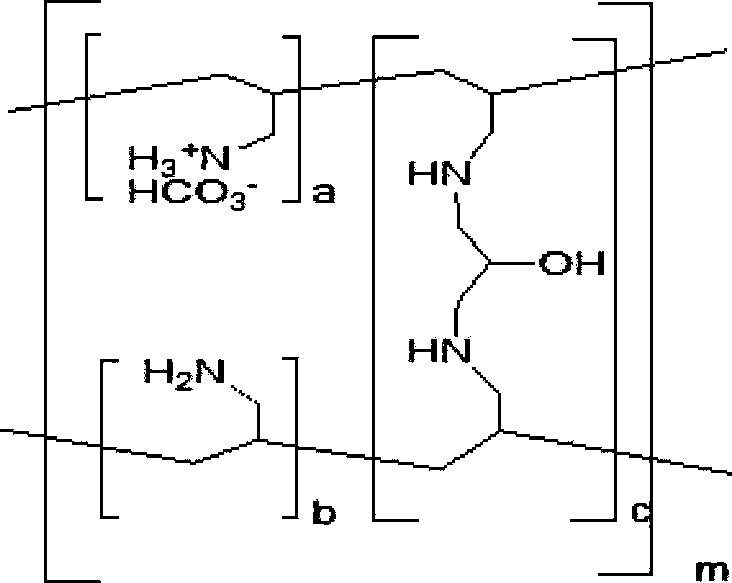
A method for treating hyperglycemia and / or reducing serum glucose levels in a patient that includes administering to the patient a therapeutically effective amount of an amine polymer is disclosed. In one embodiment, the amine polymer is aliphatic. Examples of polymers useful in an embodiment of the invention include sevelamer hydrogen chloride and colesevelam. The invention includes the use of amine polymers such as a cross-linked polymer characterized by a repeat unit having the formula: and salts and copolymers thereof, where n is a positive integer and x is zero or an integer between 1 and about 4. Also described is a use, for the manufacture of a medicament, of a polymer that lowers serum glucose.
Owner:BURKE & DONOVAN
Stable sevelamer carbonate tablet and preparation method thereof
ActiveCN107397734AHigh hardnessImprove brittlenessOrganic active ingredientsMetabolism disorderCross-linkSodium bicarbonate
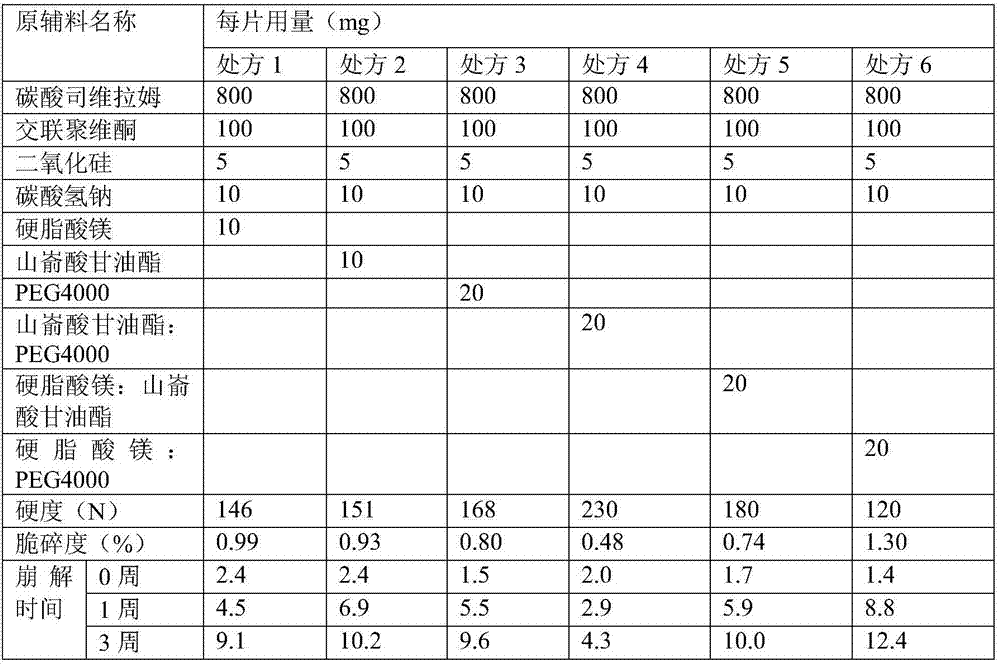
The invention relates to a stable sevelamer carbonate composition which contains sevelamer carbonate, cross-linked polyvinylpyrrolidone, silicon dioxide, sodium bicarbonate, polyethylene glycol 4000 and glyceryl behenate. The composition has the characteristics of good formability and long-term storage stability.
Owner:CP PHARMA QINGDAO CO LTD
Sevelamer carbonate crude drug for preparing tablets, preparation method and application thereof
ActiveCN102895204BFlat surfaceLow friabilityOrganic active ingredientsMetabolism disorderOligomerSevelamer
The invention belongs to the field of pharmacy, and relates to a sevelamer carbonate crude drug for preparing tablets, a preparation method and application of the sevelamer carbonate crude drug. The particle size of the sevelamer carbonate crude drug meets the conditions: D 0.95 is less than or equal to 180mu m, D 0.50 is less than or equal to 50mu m, and D 0.05 is more than or equal to 9mu m at the same time. The polyallylamine carbonate has the soluble oligomer (0.2%) within the particle size range. The tablets prepared from the sevelamer carbonate crude drug have the advantages of being smooth in surfaces, good in hardness and low in friability.
Owner:NANJING LIFENERGY R & D +1
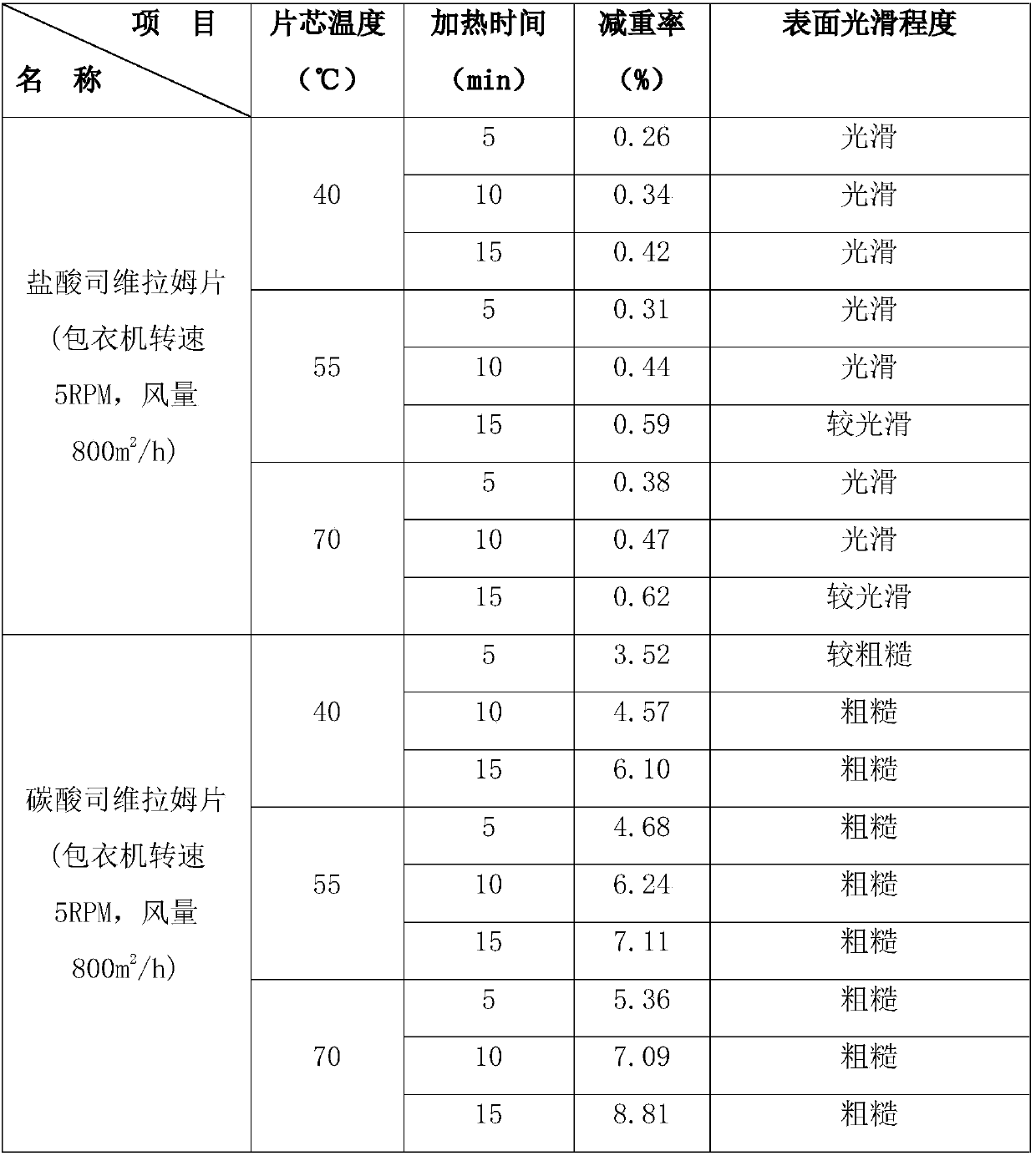
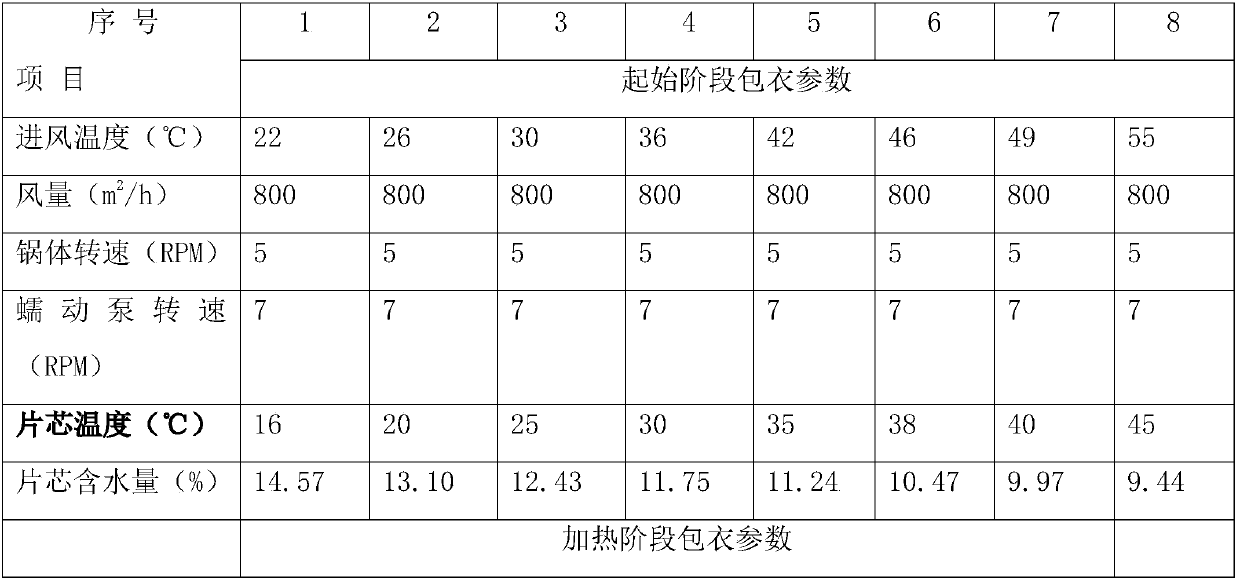
Method for coating sevelamer carbonate tablets
InactiveCN107929254AInhibit powder sheddingPrevent moisture absorptionOrganic active ingredientsDrageesTime controlSevelamer
The invention discloses a method for coating sevelamer carbonate tablets. The method comprises the following steps: under the condition that the temperature of a tablet core is controlled to 20-40 DEGC at an initial coating stage, directly spraying a coating liquid to a sevelamer carbonate tablet core, at the same time controlling the water content of the tablet core to 7-15%, after the tablet core is coated by a layer of a coating material, heating the tablet core to 30-50 DEG C, and continuously coating the tablet core till the surface of a sevelamer carbonate tablet is smooth. By adoptingthe method, the problems that powder drops from the sevelamer carbonate tablet at the coating stage, and the surface of the sevelamer carbonate tablet is rough and has hidden spots, are very well solved.
Owner:南京恒生制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com