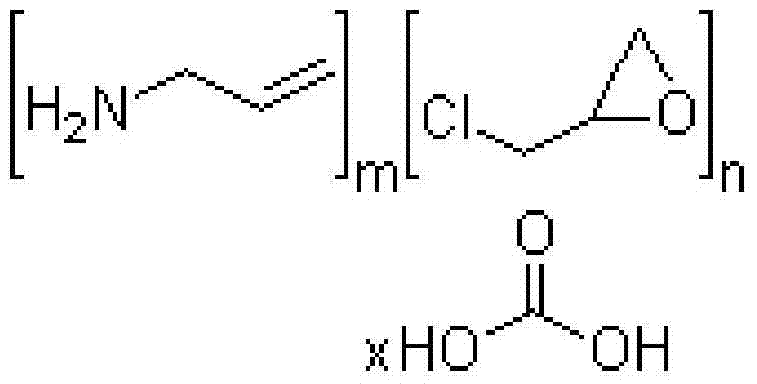
Sevelamer carbonate tablet and preparation method thereof
A technology of sevelamer carbonate tablet and sevelamer carbonate, which is applied in the field of pharmaceutical research and development, can solve problems such as long disintegration time, and achieve the effect of stable disintegration time and high effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
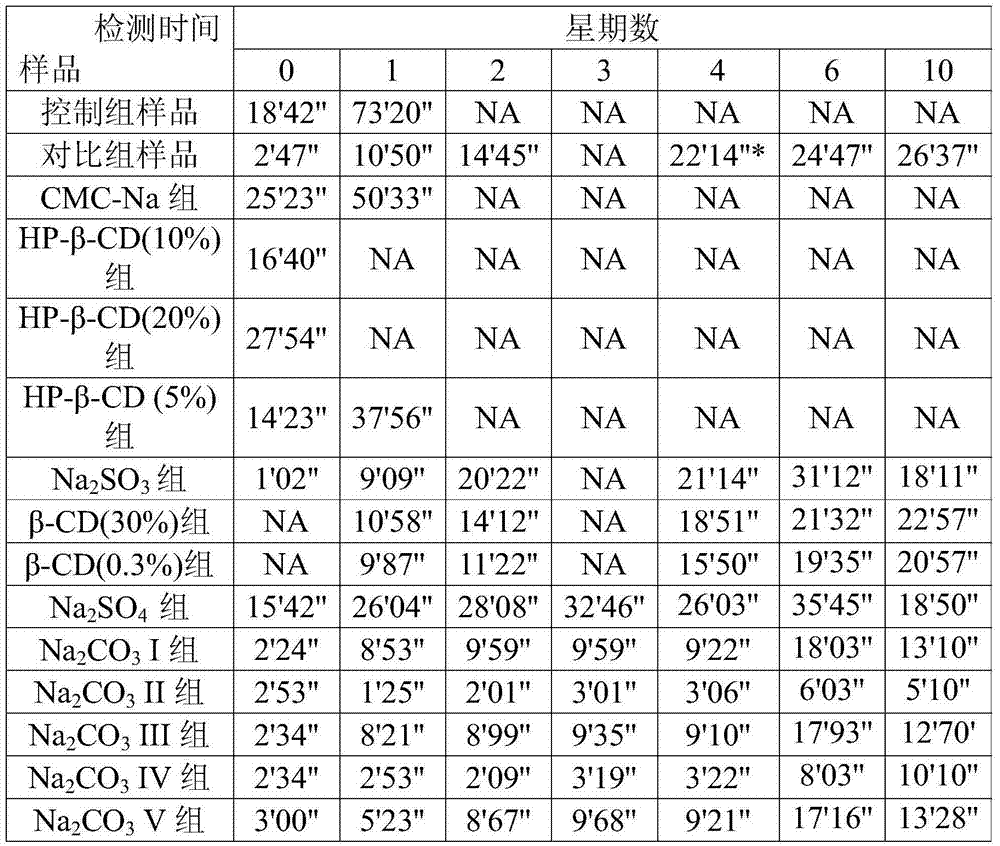
Embodiment 1
[0020] Calculated with the prescription amount of sevelamer carbonate as 800mg, mix 16.25% prescription amount of MCC (microcrystalline cellulose), 7% prescription amount of water, and 0.625% prescription amount of sodium carbonate, fully balance for at least 8 hours, and mix with After 800 mg of sevelamer carbonate (about 3% water content) was mixed uniformly, 1% of zinc stearate of the prescription was added and mixed, and then pressed into tablets to obtain sevelamer carbonate tablets.
Embodiment 2
[0022] Calculated with the prescription amount of sevelamer carbonate as 800mg, mix 16.25% prescription amount of MCC (microcrystalline cellulose), 7% prescription amount of water, and 0.3% prescription amount of β-CD (β-cyclodextrin) Uniform, fully balanced for at least 8 hours, mixed with 800 mg of sevelamer carbonate (about 3% water content), and then added with 1% of the prescription amount of zinc stearate, mixed and pressed to obtain sevelamer carbonate tablets .
Embodiment 3
[0024] The difference from Example 1 is that the consumption of sodium carbonate is 0.312% of the sevelamer carbonate recipe quantity, and the remaining steps are the same as in Example 1 to obtain sevelamer carbonate tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com