Sevelamer carbonate dry suspension for oral administration
The technology of sevelamer carbonate and dry suspension, which is applied in the field of pharmaceutical preparations, can solve the problems of poor compressibility, troublesome medication, large tablet volume, etc., and achieve the effects of improving compliance and reducing the number of times of taking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
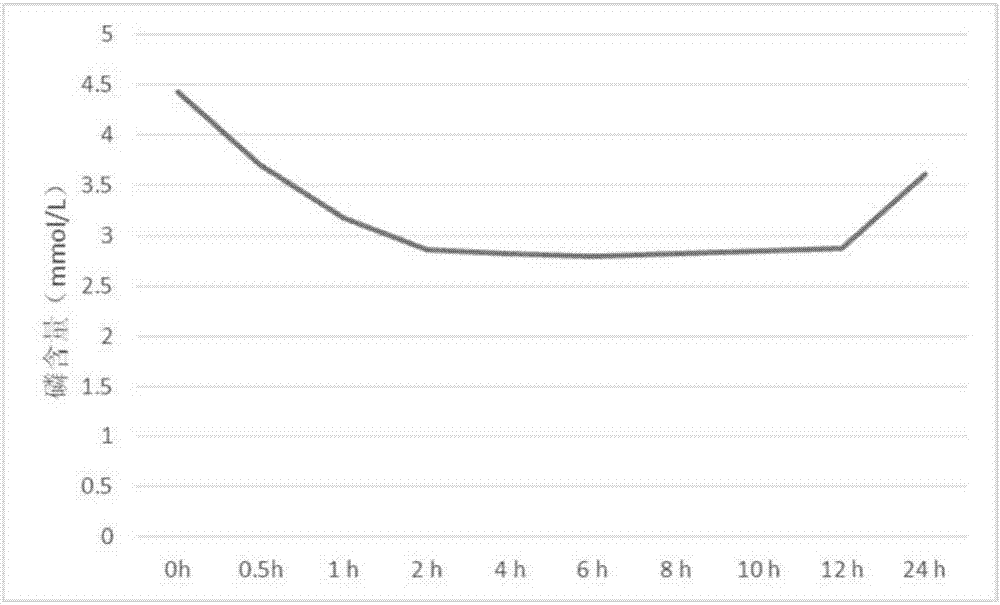
Embodiment 1
[0028] Sevelamer carbonate 180g, hydroxypropyl methylcellulose 210g, microcrystalline cellulose 150g, gum arabic 30g, magnesium stearate 24g, sucrose 3g and titanium dioxide 3g.
[0029] Pass sevelamer carbonate, hydroxypropyl methylcellulose, microcrystalline cellulose, gum arabic and magnesium stearate through 80 mesh sieves respectively, and sucrose and titanium dioxide pass through 60 mesh sieves respectively; Ram, suspending agent and filler, add water and mix evenly to prepare soft material; weigh the binder, glidant, flavoring agent and coloring agent of the formula and add them to the above soft material to make carbonic acid Sevelamer wet granules, dry sub-packaging to obtain sevelamer carbonate dry suspension.
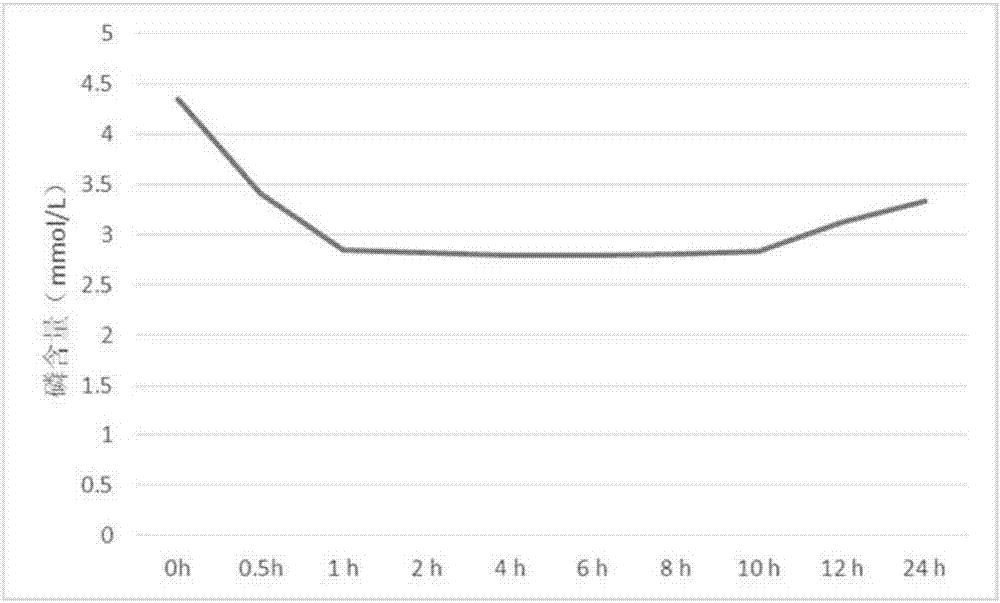
Embodiment 2
[0031] Sevelamer carbonate 300g, hydroxypropyl methylcellulose 150g, microcrystalline cellulose 90g, gum arabic 42g, magnesium stearate 12g, sucrose 5g and titanium dioxide 5g.
[0032] Pass sevelamer carbonate, hydroxypropyl methylcellulose, microcrystalline cellulose, gum arabic and magnesium stearate through 80 mesh sieves respectively, and sucrose and titanium dioxide pass through 60 mesh sieves respectively; Ram, suspending agent and filler, add water and mix evenly to prepare soft material; weigh the binder, glidant, flavoring agent and coloring agent of the formula and add them to the above soft material to make carbonic acid Sevelamer wet granules, dry sub-packaging to obtain sevelamer carbonate dry suspension.
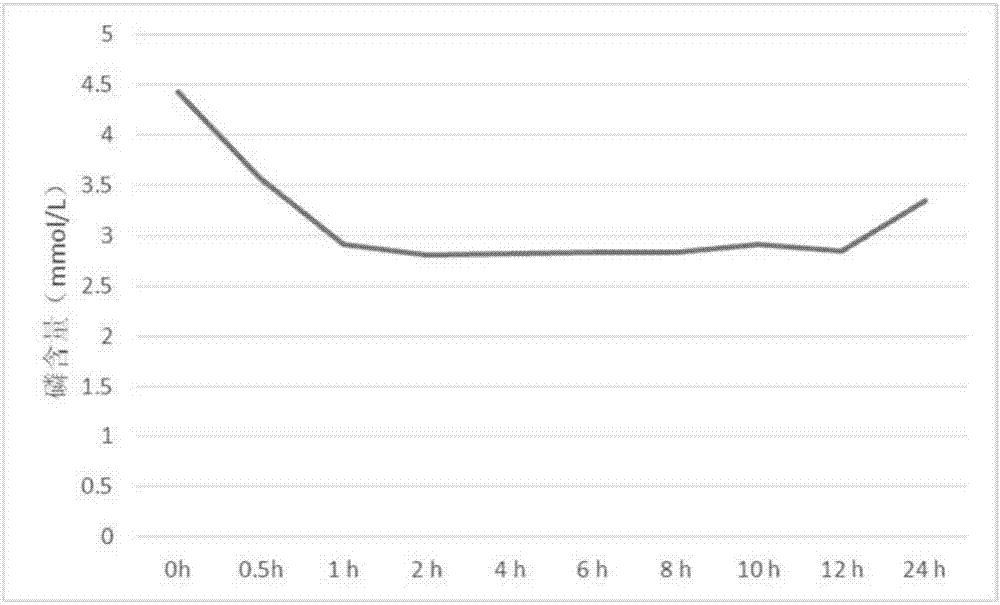
Embodiment 3
[0034] Sevelamer carbonate 240g, hydroxypropyl methylcellulose 180g, microcrystalline cellulose 120g, gum arabic 30g, magnesium stearate 27g, sucrose 1.5g and titanium dioxide 1.5g.
[0035] Pass sevelamer carbonate, hydroxypropyl methylcellulose, microcrystalline cellulose, gum arabic and magnesium stearate through 80 mesh sieves respectively, and sucrose and titanium dioxide pass through 60 mesh sieves respectively; Ram, suspending agent and filler, add water and mix evenly to prepare soft material; weigh the binder, glidant, flavoring agent and coloring agent of the formula and add them to the above soft material to make carbonic acid Sevelamer wet granules, dry sub-packaging to obtain sevelamer carbonate dry suspension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com