Sevelamer carbonate effervescent tablets and preparation method thereof
The technology of sevelamer carbonate and sevelamer carbonate is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery, which can solve the problems of inconvenient tablet carrying, poor compressibility, and troublesome medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
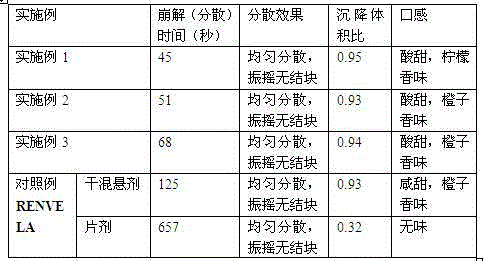
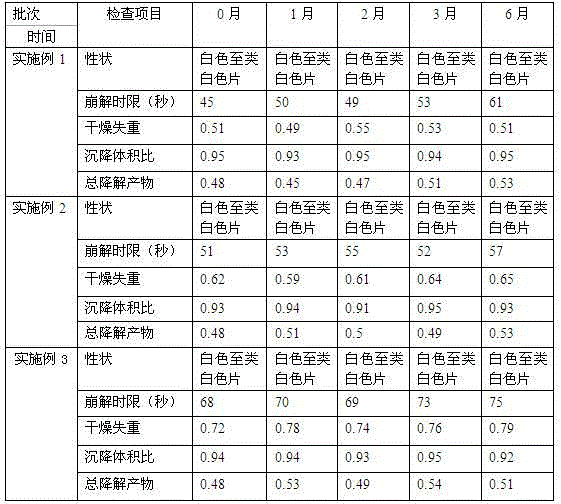
Examples
Embodiment 1
[0027] Prescription composition:
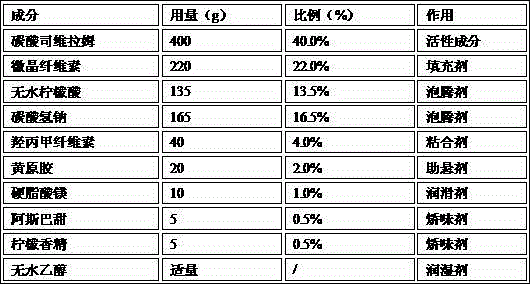
[0028] Table 1 The prescription dosage table of Example 1 (1000 batches)
[0029]
[0030] The preparation process is as follows:
[0031] 1) Pulverize sevelamer carbonate, control the particle size at 10-50 microns, and pulverize the remaining materials through an 80-mesh sieve for subsequent use;
[0032] 2) Weigh the prescribed amount of adhesive, add absolute ethanol, stir evenly, and prepare a solution with a solid content of 5% for subsequent use;
[0033] 3) Put the sevelamer carbonate, effervescent agent, and filler prepared in step 1) in the shear granulator, turn on the granulator, and spray into the 5% binder anhydrous ethanol solution prepared in step 2) Thoroughly mix to obtain mixture I;
[0034] 4) Granulate the mixture I in step 3) through a 16-30 mesh screen, dry at 55-60°C until the water content is less than 1.0%, and granulate with a 18-24 mesh screen to obtain mixture II;
[0035]5) Put the mixture II in st...
Embodiment 2
[0038] Prescription composition:
[0039] Table 2 The prescription dosage table of embodiment 2 (1000 batches)
[0040] Element Dosage (g) Proportion(%) effect Sevelamer Carbonate 750 50.0% active ingredient lactose 180 12.0% filler tartaric acid 150 10.0% Effervescent sodium bicarbonate 170 11.3% Effervescent Povidone K30 120 8.0% Adhesive Propylene glycol alginate 45 3.0% suspending agent polyethylene glycol 8000 75 5.0% lubricant Acesulfame K 5 0.33% flavoring agent Sweet Orange Flavor 5 0.33% flavoring agent Absolute ethanol Appropriate amount / D
[0041] The preparation process is the same as in Example 1.
Embodiment 3
[0043] Prescription composition:
[0044] Table 3 The prescription dosage table of embodiment 3 (1000 batches)
[0045] Element Dosage (g) Proportion(%) effect Sevelamer Carbonate 1400 70.0% active ingredient Mannitol 300 15.0% filler malic acid 100 5.0% Effervescent Sodium carbonate 100 5.0% Effervescent Povidone K30 29 1.5% Adhesive Propylene glycol alginate 20 1.0% suspending agent stearic acid 50 2.5% lubricant Stevioside 0.5 0.025% flavoring agent Sweet Orange Flavor 0.5 0.025% flavoring agent Absolute ethanol Appropriate amount / D
[0046] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com