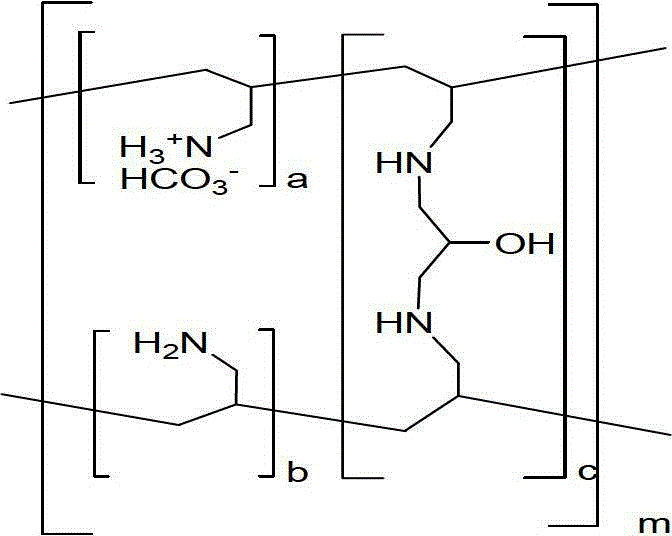
Sevelamer carbonate medical tablet composition and preparation method thereof
A carbonic acid sevelamer tablet, a technology of carbonic sevelamer, which is applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., and can solve the problems of many conditions, inconvenient use, inability to achieve effects, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
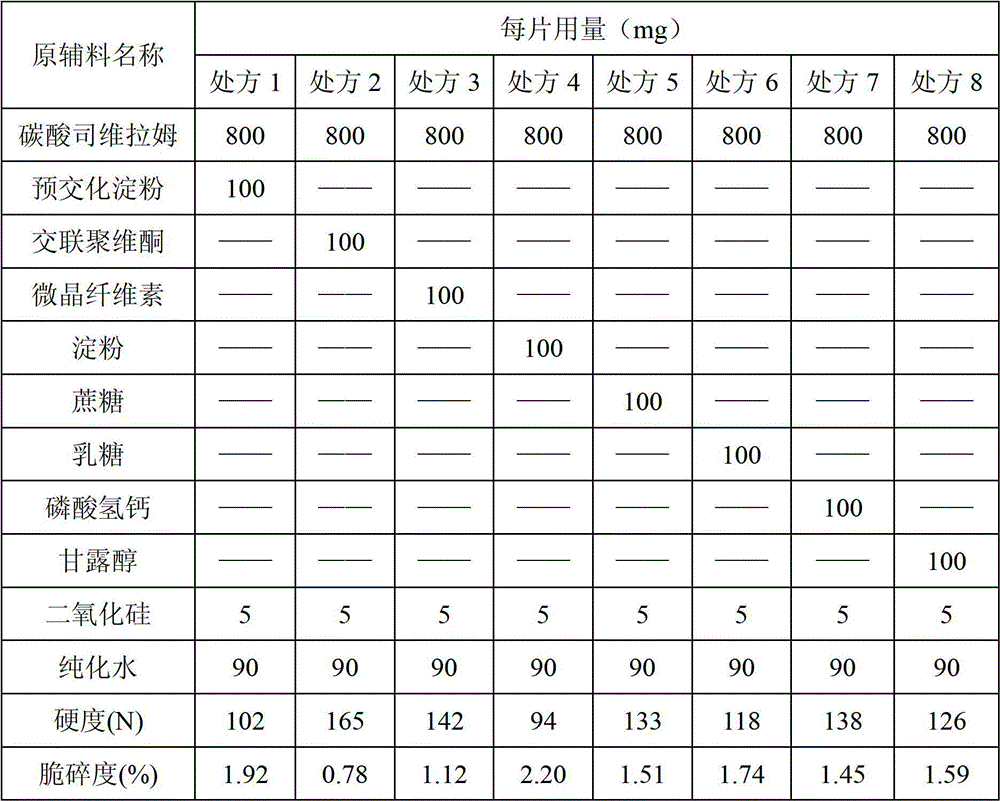
[0031] Prepare tablet according to prescription in table 1 and following process, and tablet measures hardness and friability, is listed in the following table:
[0032] The raw and auxiliary materials, except silicon dioxide, are mixed in a wet mixing granulator according to the above ratio, mixed evenly with water, granulated with a swing granulator at 20 mesh, and then silicon dioxide is added to the granules, mixed evenly, and pressed into Vegetable tablets, determine the hardness and friability of the plain tablets.
[0033] Table 1
[0034]
[0035] The study found that using different types of excipients mixed with sevelamer carbonate for granulation and tableting, only when crospovidone was used as the main filler, the hardness and friability of plain tablets could meet the basic requirements of tablets. The friability of plain tablets obtained from other types of auxiliary materials cannot meet the requirement of less than 1%.
Embodiment 2
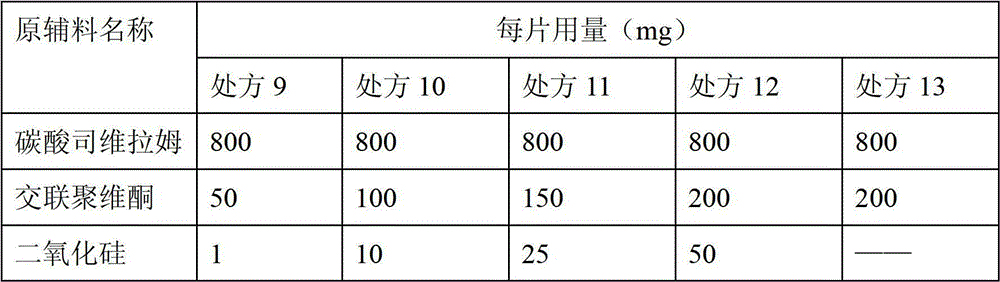
[0037] Preparation of tablets according to the ratio in Table 2: mix sevelamer carbonate with crospovidone, add purified water to mix, granulate with a 20-mesh swing granulator, mix the granules with silicon dioxide, and press into plain tablets, and determine the hardness and friability of the plain tablets.
[0038] Table 2
[0039]
[0040]
[0041] The results of comparative experiments show that: without adding silicon dioxide and using in combination with crospovidone, the friability and hardness of the prepared plain tablets cannot meet the requirements; moreover, adding 50-200 mg of crospovidone and 1-50 mg With each formulation of silicon dioxide, the plain tablets prepared can meet the requirements of friability and hardness.
Embodiment 3
[0043] Tablets are prepared according to the ratio in Table 3: mix sevelamer carbonate and crospovidone, add purified water to mix, granulate with a 20-mesh swing granulator, and then mix the granules with silicon dioxide and stearic acid Magnesium, stearic acid, glyceryl behenate, PEG6000, talcum powder, etc. were mixed evenly, pressed into plain tablets, and the hardness and friability of the plain tablets were measured.
[0044] table 3
[0045]
[0046] The experimental results show that adding a lubricant to the basic formulation can improve the fluidity of the intermediate granules without affecting the hardness and friability of plain tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com