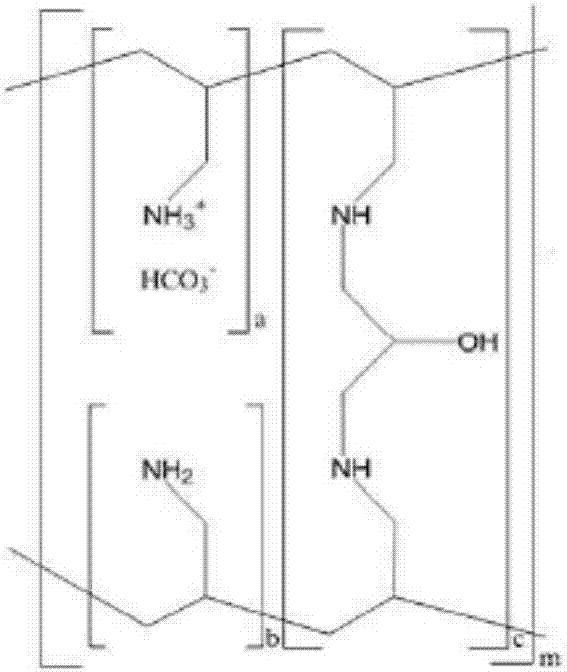
Stable sevelamer carbonate tablet and preparation method thereof
A stable technology of sevelamer carbonate, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, which can solve the problems of poor formability of tablets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
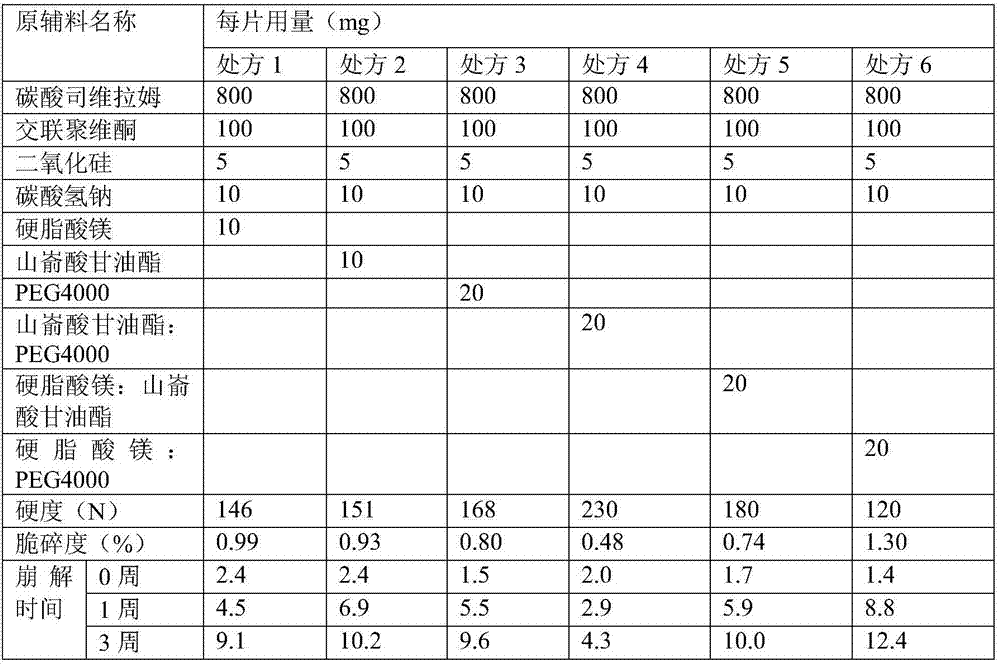
[0024] Tablets were prepared according to the prescription in Table 1 and the following process, and the hardness, friability and disintegration time of the tablets were determined.
[0025] After mixing sevelamer carbonate with sodium bicarbonate, mix it with crospovidone; add about 6-13 parts by weight of water, and use a swing granulator to granulate; add silicon dioxide and lubricant to the granules, mix and press to form Plain tablets, determination of hardness and friability of plain tablets, and tablet core disintegration at pH 1.2, with dish and sieve at 0, 1, 3 weeks for tablets stored at 60 Average time (minutes).
[0026] Table 1
[0027]
[0028] Studies have found that after adding 0.5-2 parts by weight of sodium bicarbonate, the hardness and brittleness and hardness of the pharmaceutical composition do not change much, but the storage stability is greatly affected. When using a single magnesium stearate, glyceryl behenate Or when PEG4000 is used as a lubrica...
Embodiment 2
[0030] Depend on Sevelamer carbonate tablets prepared with glyceryl behenate and PEG4000 as lubricants showed good stability. The next experiment will further optimize the ratio of glyceryl behenate and PEG4000, and determine the hardness, friability Friability and average tablet core disintegration time (minutes) at pH 1.2, with dish and sieve, at 0, 1, and 3 weeks for tablets stored at 60°C.
[0031] Tablets were prepared according to the proportioning in Table 2, and the preparation method was the same as in Example 1.
[0032] The results showed that when the ratio of polyethylene glycol 4000 and glyceryl behenate was 10:1-20:1, the stability of the tablet was better, and when the ratio of the two was 1:1, the stabilizer took second place. The applicant screened the lubricant through a large number of experiments. During the screening process, it was unexpectedly found that when the lubricant is a combination of polyethylene glycol 4000 and glyceryl behenate, the stabili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com