Environment-friendly synthesis method for cinacalcet
A technology of green synthesis and compound, which is applied in the field of green synthesis of Cinacase to achieve the effect of reducing pollution, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
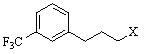
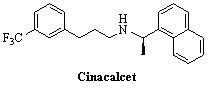
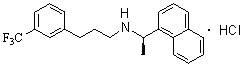
[0042] Add 0.6g R-(+)-1-naphthylethylamine, 0.86g [3-(3'-trifluoromethyl)phenyl]-1-chloropropane, 0.28g sodium hydroxide 8ml of aqueous solution and 0.06g of tetrabutylammonium bromide, stirred, and reacted at 70-75°C for 4h. Cool and extract with dichloromethane. The extract was washed with dilute hydrochloric acid, saturated sodium bicarbonate and saturated brine in sequence, dried over anhydrous sodium sulfate, filtered, and the solvent was removed to obtain cinacalcet. Pale yellow liquid, the yield is 82.1%, [α] D =-8.6 o (C=1, methanol).
Embodiment 2
[0044] Add 0.6g R-(+)-1-naphthylethylamine, 0.86g [3-(3'-trifluoromethyl)phenyl]-1-chloropropane, 0.40g potassium hydroxide 8ml of aqueous solution and 0.09g of tetrabutylammonium iodide, stirred, and reacted at 70-75°C for 4h. Cool and extract with dichloromethane. The extract was washed with dilute hydrochloric acid, saturated sodium bicarbonate and saturated brine in sequence, dried over anhydrous sodium sulfate, filtered, and the solvent was removed to obtain cinacalcet. Pale yellow liquid, yield 83.0%, [α] D =-9.2 o (C=1, methanol).
Embodiment 3
[0046] Add 0.6g R-(+)-1-naphthylethylamine, 0.93g [3-(3'-trifluoromethyl)phenyl]-1-chloropropane, and 1.1g of sodium carbonate in a 50ml three-necked flask successively. 10ml of aqueous solution and 0.09g of PEG6000 were stirred and reacted at 95-100°C for 24h. Cool and extract with dichloromethane. The extract was washed with dilute hydrochloric acid, saturated sodium bicarbonate and saturated brine in sequence, dried over anhydrous sodium sulfate, filtered, and the solvent was removed to obtain cinacalcet. Pale yellow liquid, the yield is 78.5%, [α] D =-8.8 o (C=1, methanol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com