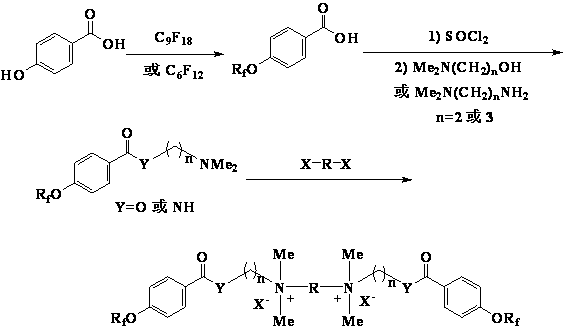
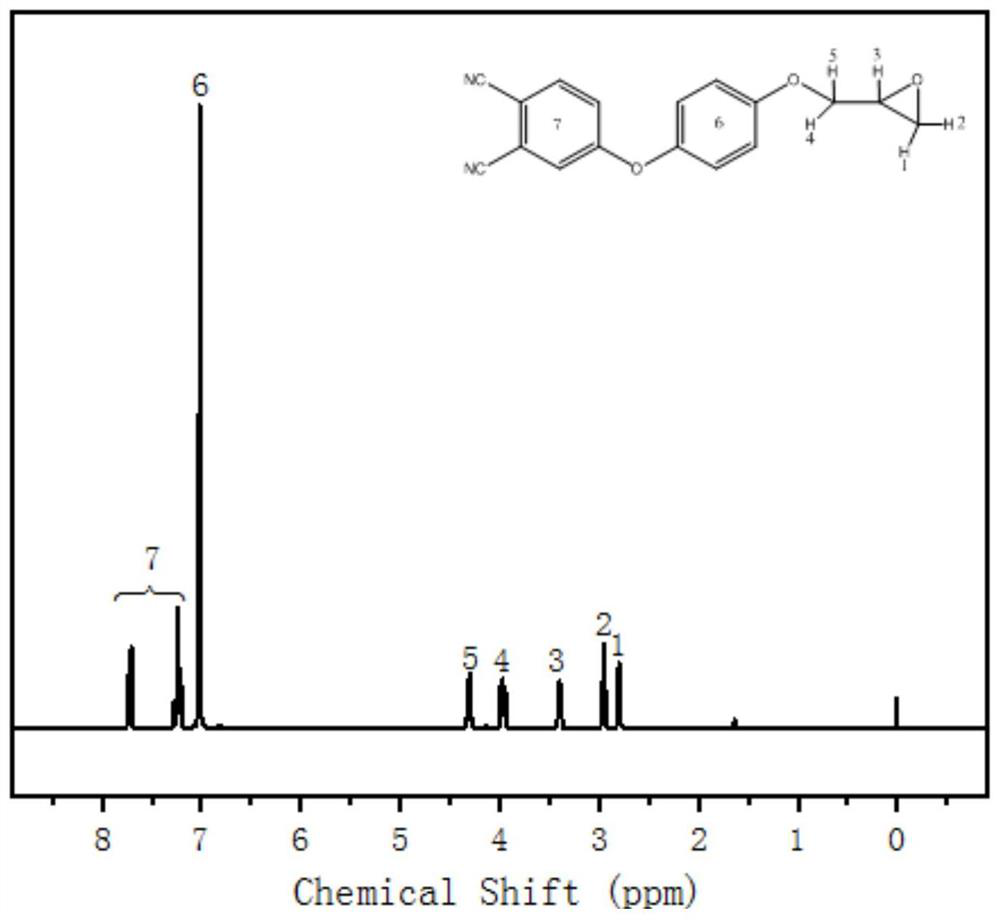
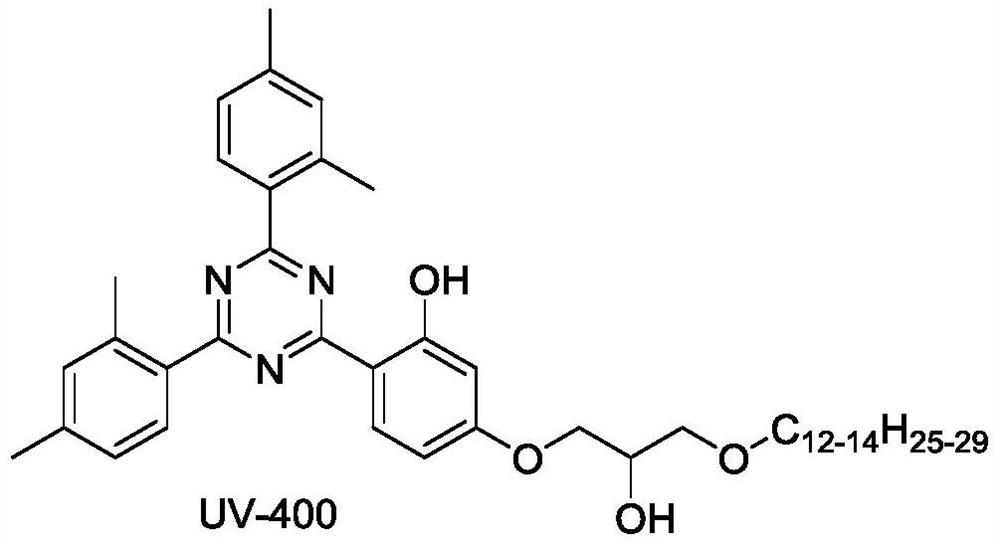
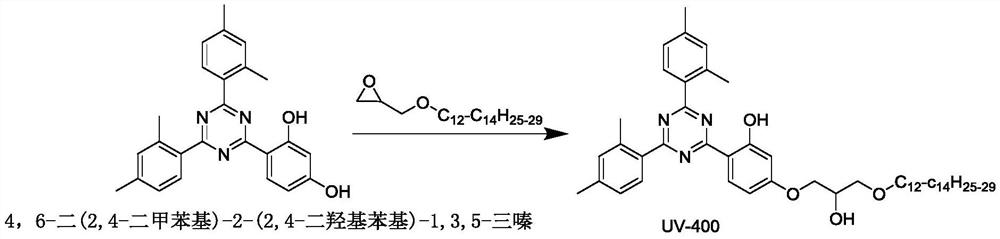
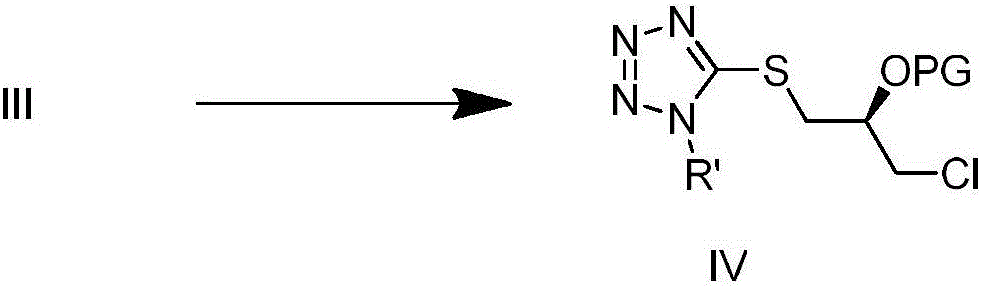
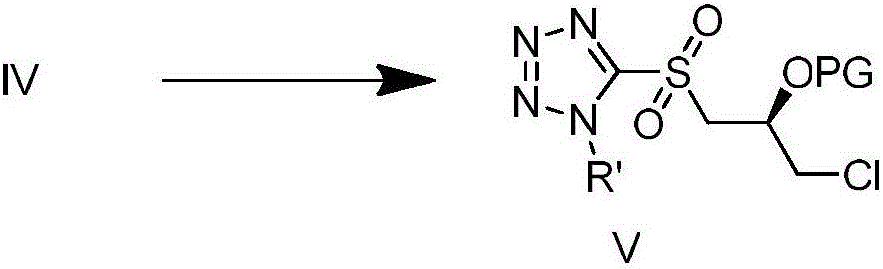
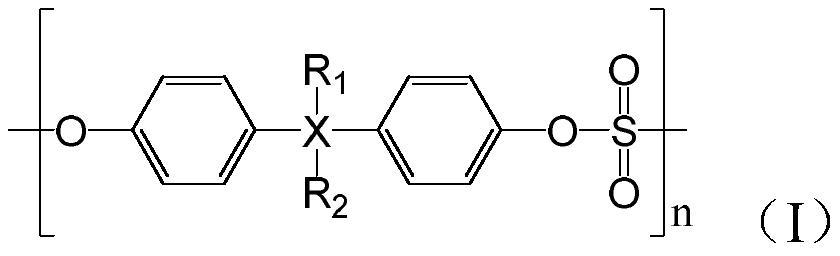
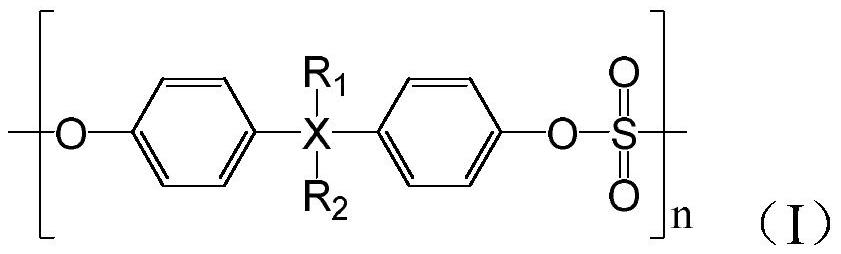
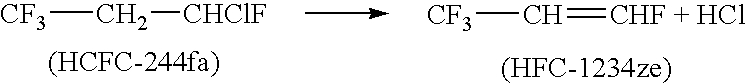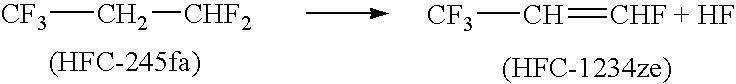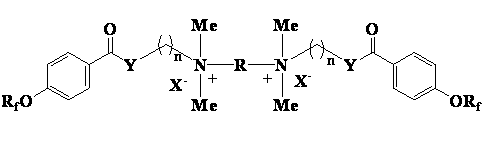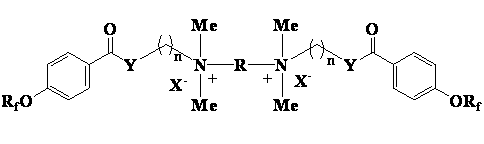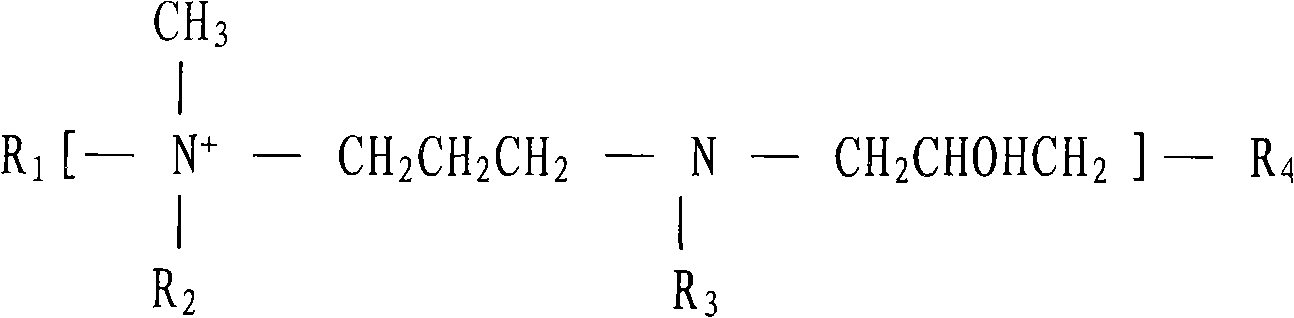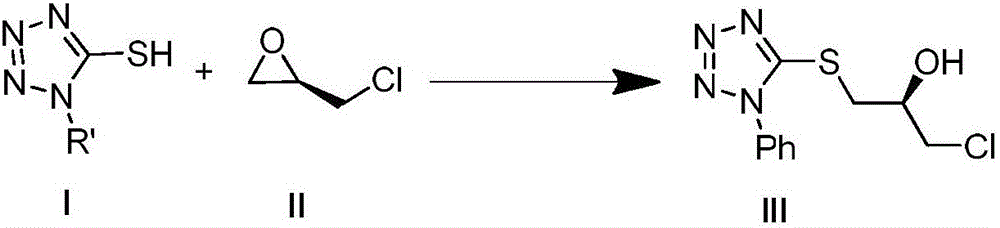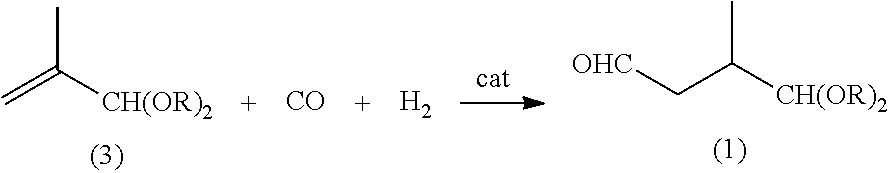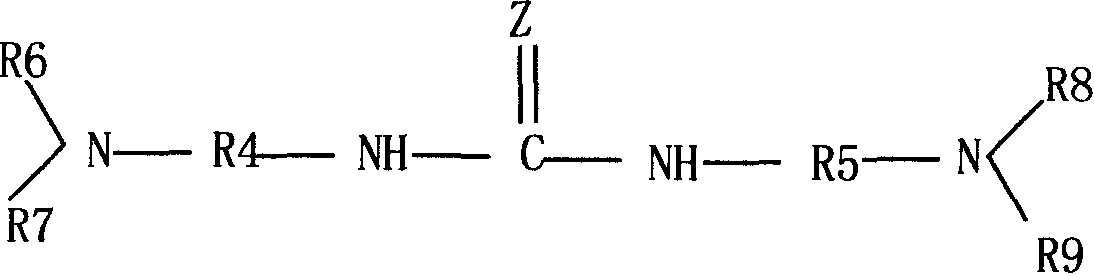Patents
Literature
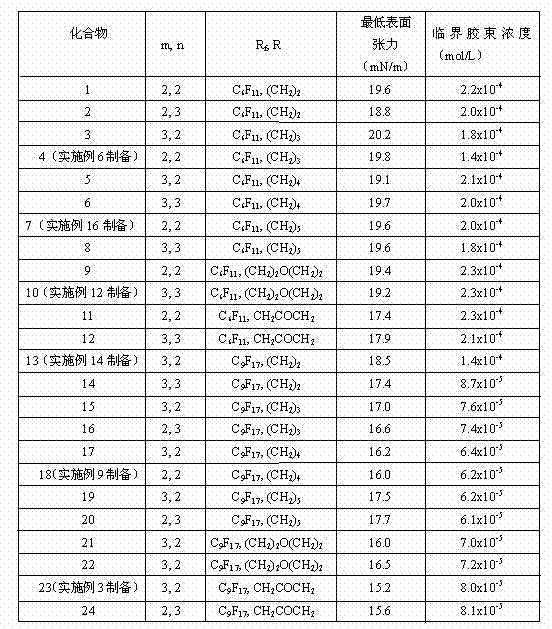
31 results about "Halopropane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Halopropane (synonym FHD-3, trade name Tebron) is a halocarbon drug which was investigated as an inhalational anesthetic but was never marketed. Its clinical development was terminated due to a high incidence of cardiac arrhythmias in patients, similarly to the cases of teflurane and norflurane.
Process for producing fluoropropenes
InactiveUS7230146B2Simplifies isolationPreparation by hydrogen halide split-offPreparation by halogen halide additionHalogenHydrogen
Dehydrohalogenation processes for the preparation of fluoropropenes from corresponding halopropanes, in which the fluoropropenes have the formula CF3CY═CXNHP, wherein X and Y are independently hydrogen or a halogen selected from fluorine, chlorine, bromine and iodine; and N and P are independently integers equal to 0, 1 or 2, provided that (N+P)=2.
Owner:HONEYWELL INT INC
Processes for the preparation of 2-chloro-1,1,1,2,3,3,3-heptafluoropropane, hexafluoropropene and 1,1,1,2,3,3,3-heptafluoropropane
InactiveUS7129383B2Preparation by dehalogenationPreparation by hydrogen halide split-offCrystalline oxideHalohydrocarbon
Owner:EI DU PONT DE NEMOURS & CO
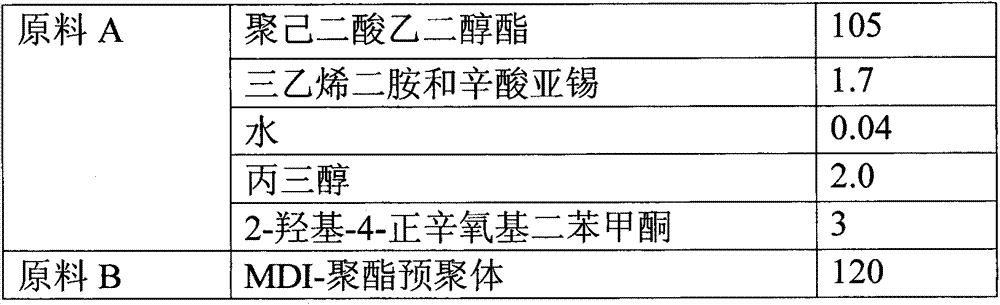
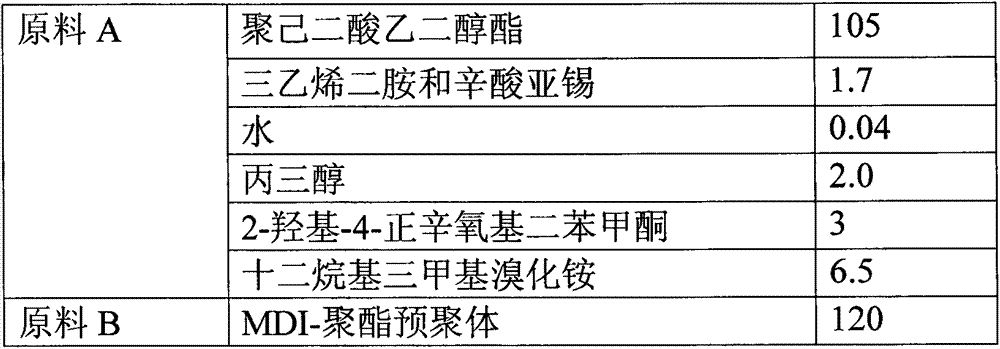
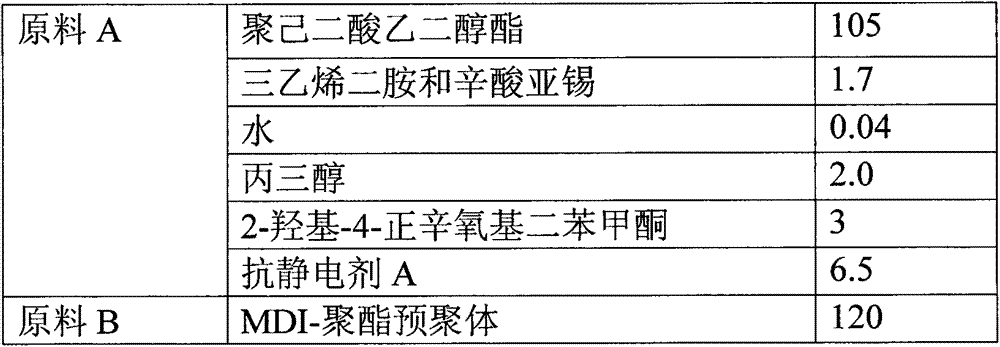
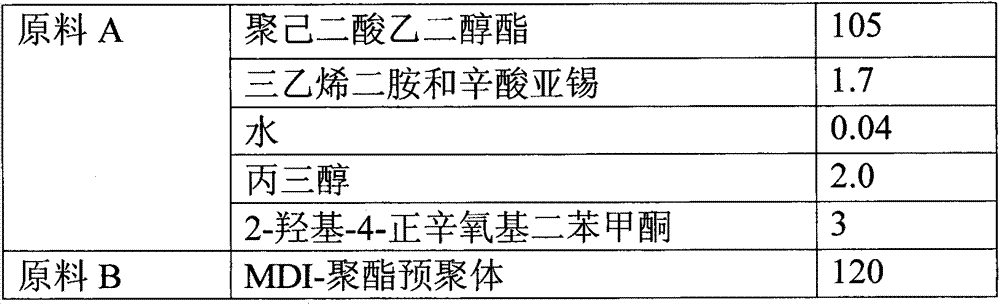
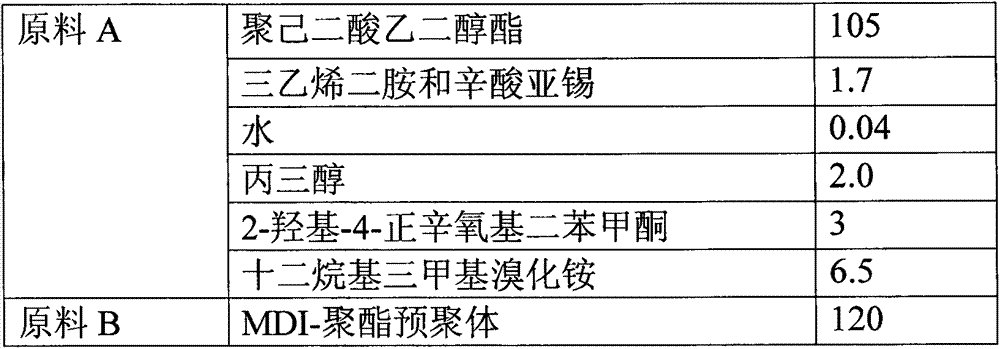
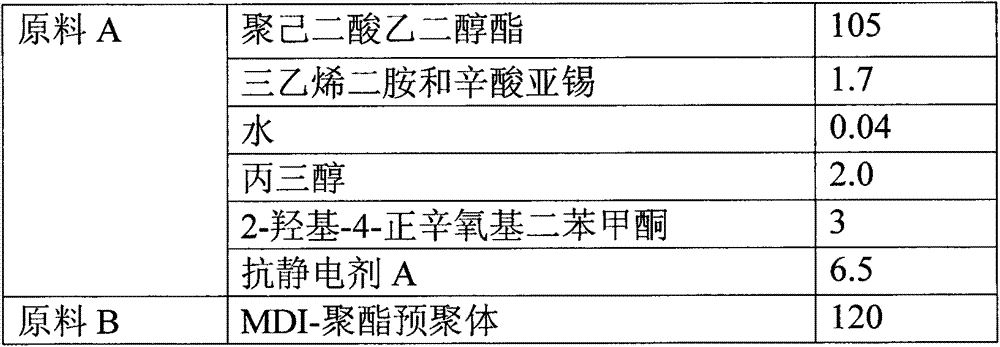
Reaction type antistatic agent, preparation method and durable antistatic polyurethane material
ActiveCN103113575AGood dissipation performanceIncreased durabilitySolesOrganic compound preparationEpoxyAlcohol
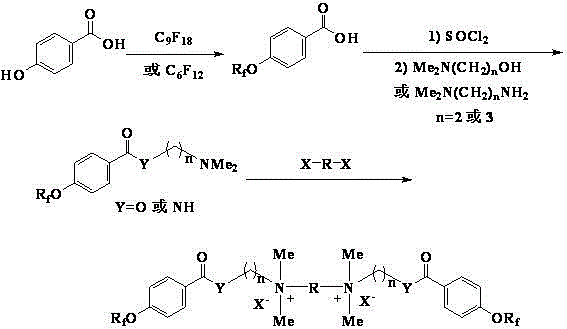
The invention provides a reaction type antistatic agent. The antistatic agent is generated by reacting an intermediate which is synthesized under the alkaline condition based on polyethylene glycol and epoxy halopropane as raw materials with trialkylamine haloid, wherein the molecular weight of polyethylene glycol is 400-2,000. The invention also provides a preparation method of the reaction type antistatic agent, which comprises the following steps of: (1) synthesizing the intermediate at 40-60 DEG C under the alkaline condition by taking polyethylene glycol and epoxy halopropane as the raw materials; and (2) reacting the intermediate with trialkylamine haloid in an alcohol solvent at 20-40 DEG C to obtain the reaction type antistatic agent. The invention also provides an antistatic polyurethane material which is prepared by mixing and pouring a raw material A and a raw material B, wherein the raw material A comprises polyhydric alcohol, a catalyst, a foaming agent, a chain extender and the reaction type antistatic agent; and the raw material B is isocyanate.
Owner:SHENZHEN SELEN SCI & TECH CO LTD
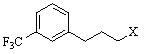
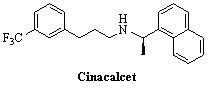
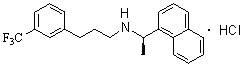
Environment-friendly synthesis method for cinacalcet
InactiveCN101941911AReduce pollutionSimple and fast operationAmino preparation by functional substitutionPtru catalystMeth-
The invention discloses an environment-friendly synthesis method for cinacalcet. The cinacalcet is prepared by reacting R(+)-1-naphthyl ethamine and [3-(3'-trifluoromethyl)phenyl]-1-halopropane or [3-(3'-trifluoromethyl)phenyl]-1-propylsulfonate serving as raw materials with water serving as medium under the action of a phase-transfer catalyst. The process has the advantages of reducing the cost, along with high yield, no any organic solvent during the reaction, and low environmental pollution, and thus is a synthesis method suitable for industrialization.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and preparation method of cationic type gemini fluorinated surfactant
ActiveCN102908937AHigh reactivityReactive and expensiveOrganic compound preparationTransportation and packagingBenzoic acidPolymer science
The invention discloses a cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and a preparation method of the cationic type gemini fluorinated surfactant. The preparation method comprises the following steps of: with the perfluorinated nonene or perfluorinated hexene as a raw material, condensing the perfluorinated nonene or perfluorinated hexene with p-hydroxybenzoic acid, and carrying out chlorination on a matter obtained after condensation and thionyl chloride to prepare perfluorinated alkene oxyl benzoyl chloride; condensing the perfluorinated alkene oxyl benzoyl chloride without separation with 2-dimethylamino ethanol, 3-dimethylamino propanol, 2-dimethylamino ethylamine and 3-dimethylamino propylamine to obtain perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with tertiary amine at an alkanol part or amine part; and finally, condensing the perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with 1,2-dihaloethane, 1,3-dihalopropane, 1,4-dichlorobutane, 1,5-dichloropentane or 3-oxa-1,5-dichloropentane to prepare a quaternary ammonium gemini fluorinated surfactant. The synthesized compound is high in surface activity and is low in critical micelle concentration, has the characteristics of simpleness in synthesization, low cost and the like and has good application prospect.
Owner:江苏超至和新材料有限公司
Method for Producing 2,3,3,3-Tetrafluoropropene
ActiveUS20150080619A1Simple designOvercomes drawbackSolidificationLiquefactionHydrogen fluorideDistillation
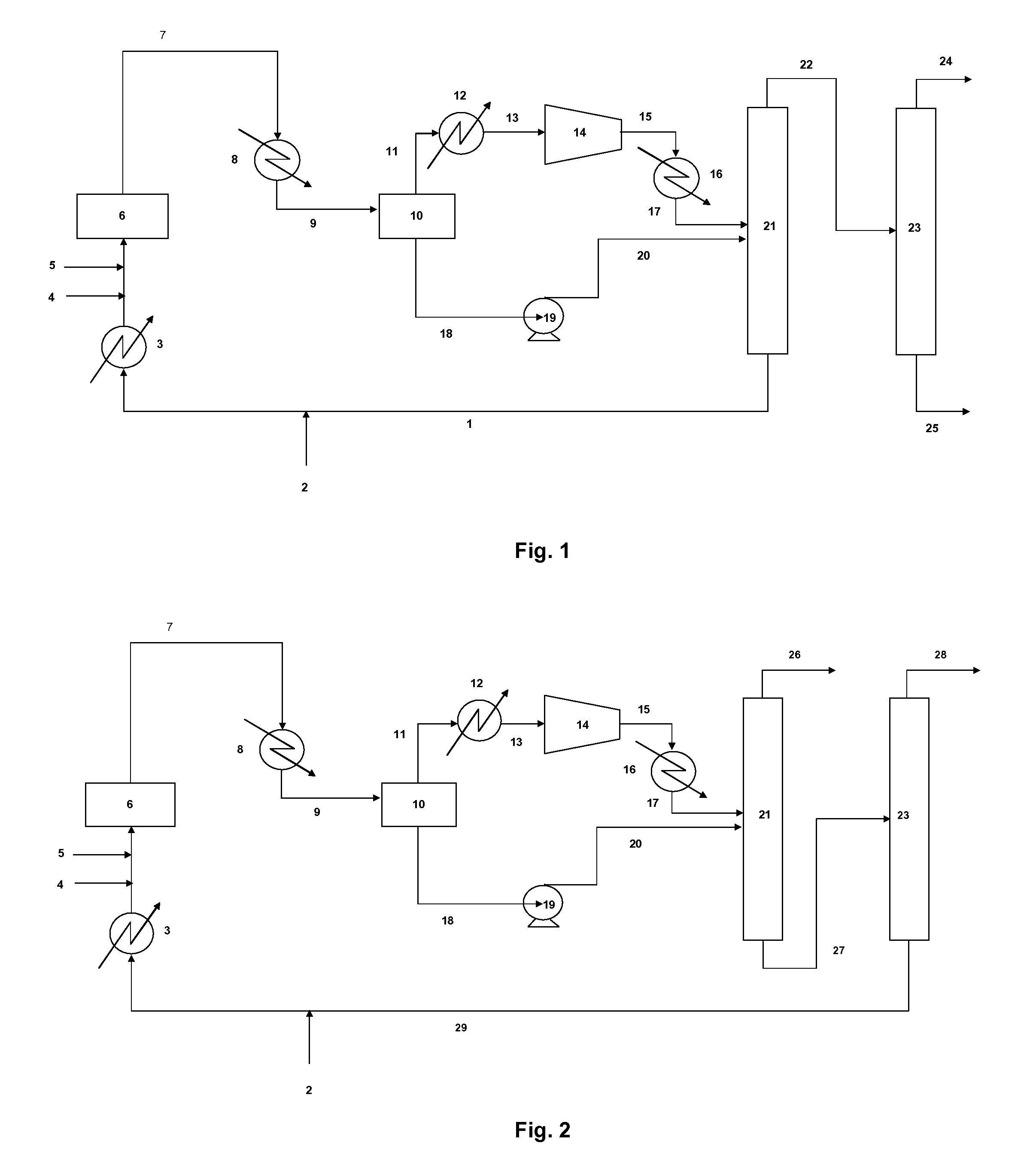
The invention concerns a method for producing 2,3,3,3-tetrafluoropropene comprising: a fluoridation reaction of a halopropane and / or halopropene into 2,3,3,3-tetrafluoropropene by means of hydrogen fluoride; the recovery of a gas stream resulting from the reaction; the cooling and partial condensation of the gas stream resulting from the reaction into a partially condensed stream; the separation of the partially condensed stream into a gas fraction and a liquid fraction; the compression of the gas fraction into a compressed gas fraction; the compression of the liquid fraction into a compressed liquid fraction; the distillation of the compressed gas fraction and compressed liquid fraction in order to provide a stream of 2,3,3,3-tetrafluoropropene, a stream of hydrochloric acid, and a stream of unreacted hydrogen fluoride. The invention also concerns an installation suitable for implementing said method.
Owner:ARKEMA FRANCE SA
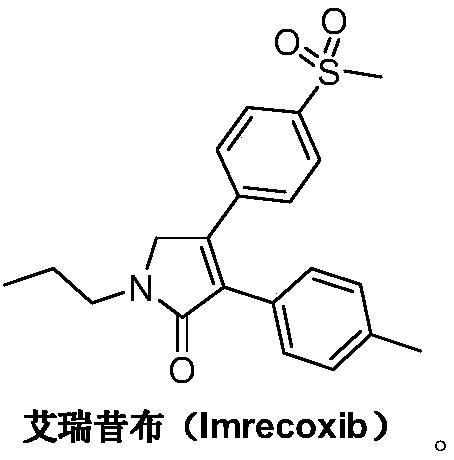
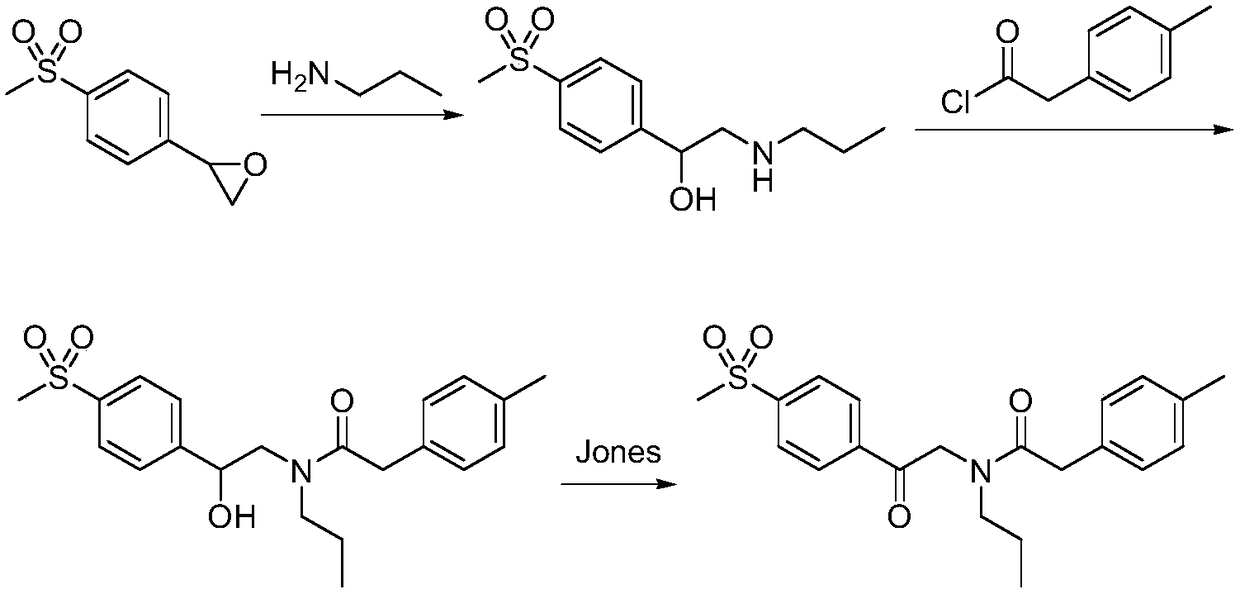
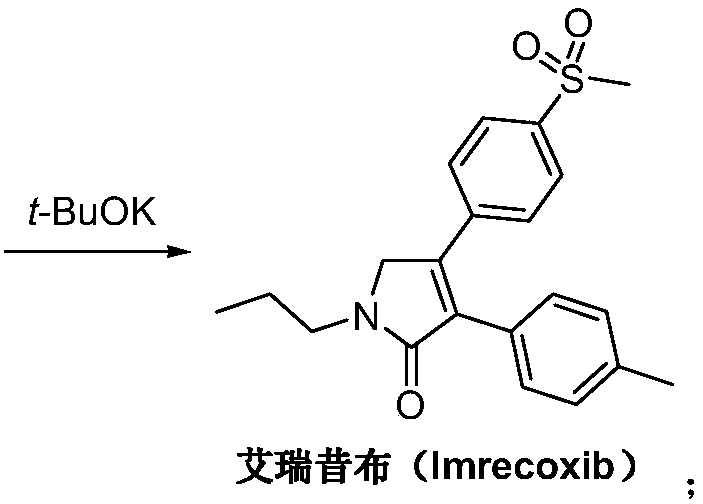
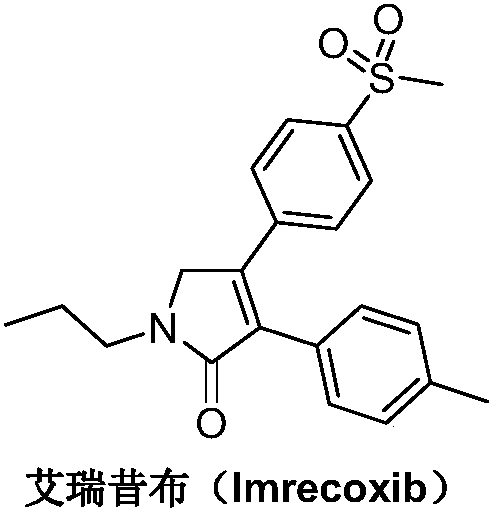
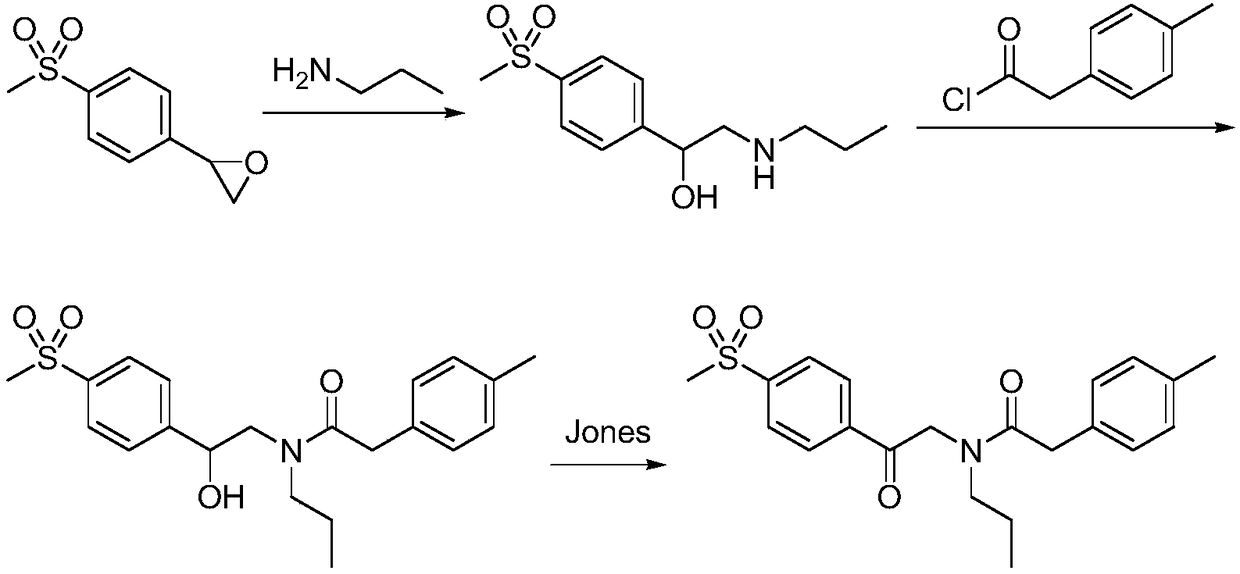
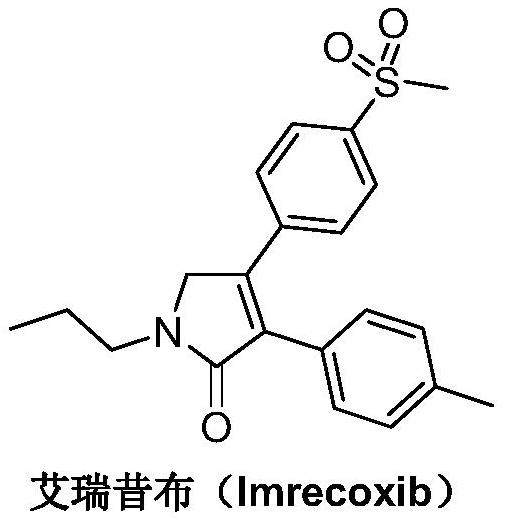
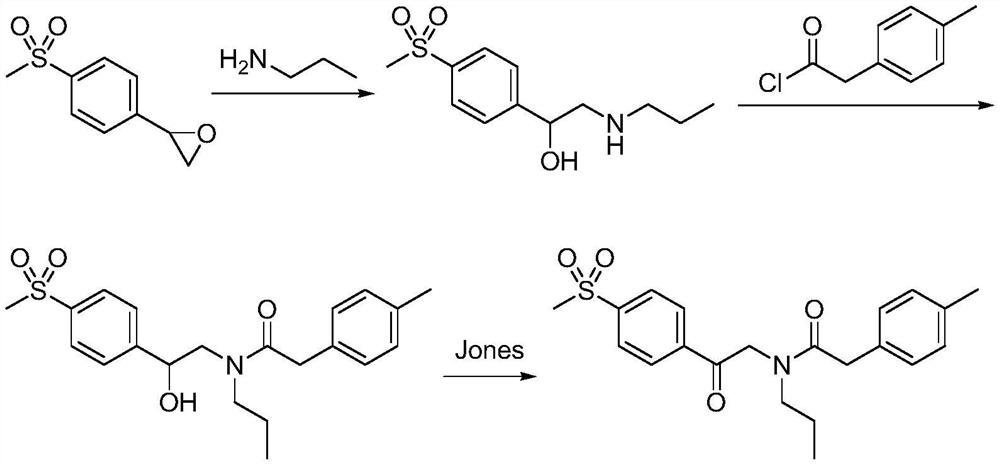
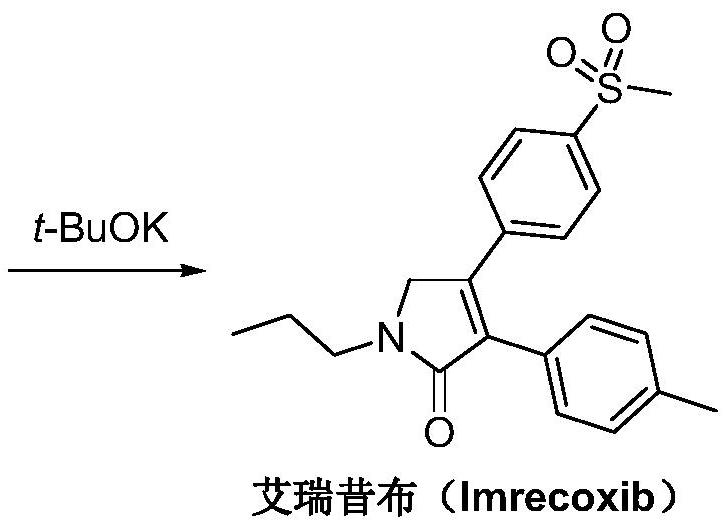
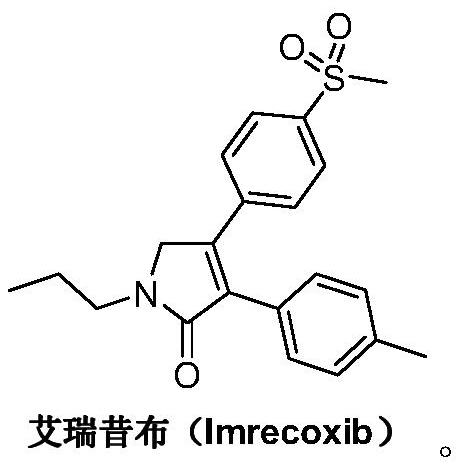
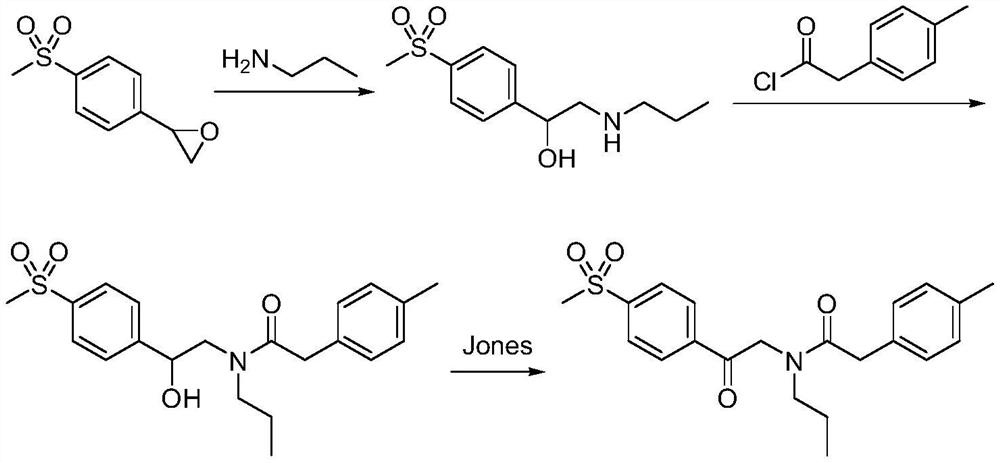
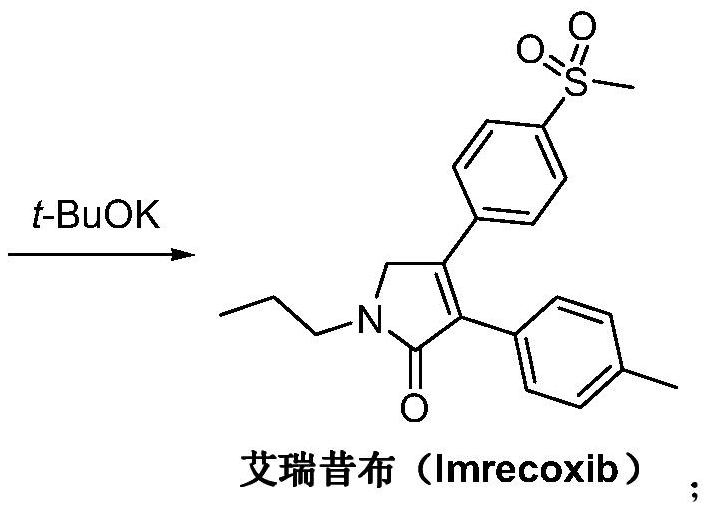
Preparation method of imrecoxib and intermediate thereof
The invention discloses a preparation method of imrecoxib and an intermediate thereof. the preparation methoc comprises the following steps: 1) carrying out an amidation reaction between 2-amino-1-p-methylsulfonyl acetophenone and p-methylphenylacetyl halide so as to obtain N-[2-oxo-2-(4-methylsulfonylphenyl)]ethyl-4-methyl phenylacetamide; 2) carrying out a condensation and cyclization reaction on N-[2-oxo-2-(4-methylsulfonylphenyl)]ethyl-4-methyl phenylacetamide so as to obtain 3-p-methyl phenyl-4-p-methylsulfonylphenyl-3-pyrrolidine-2-ketone; and 3) carrying out a substitution reaction between 3-p-methyl phenyl-4-p-methylsulfonylphenyl-3-pyrrolidine-2-ketone and 1-halopropane or hydrocarbon sulfoacid propyl ester derivative so as to obtain imrecoxib; and preparing the intermediate of imrecoxib: N-[2-oxo-2-(4-methylsulfonylphenyl)]ethyl-4-methyl phenylacetamide or 3-p-methylphenyl-4-p-methylsulfonylphenyl-3-pyrrolidine-2-ketone. The reaction process is optimized by the synthetic route. Separation in each step is simpler, purity is higher, and ideal yield of the product also can be achieved.
Owner:江苏美迪克化学品有限公司
Method for preparing mercapto polyether
Owner:BEIJING UNIV OF CHEM TECH
Alkaline environmental-friendly zinc-plating additive for changing cyanogen process into cyanogen-free process
The invention relates to an alkaline environmental-friendly zinc-plating additive for changing a cyanogen process into a cyanogen-free process. The additive comprises the following substances: at least one organic heterocyclic compound and derivative, at least one organic amine-epoxy halopropane polycondensate, wherein the molar ratio of the organic heterocyclic compound and derivative to an alicyclic amine compound and the polycondensate is 0.5-4.0:0.5-5.0. The additive overcomes the defects of severe environmental pollution caused by the conventional cyanogen zinc plating process. After sodium cyanide is stopped adding, the cyanogen zinc plating process can be gradually changed into a cyanogen-free environmental-friendly alkaline zinc plating process on the premise of not influencing normal production by adopting the zinc-plating additive.
Owner:武汉风帆电化科技股份有限公司
Processes for the preparation of 2-chloro-1,1,1,2,3,3,3-heptafluoropropane, hexafluoropropene and 1,1,1,2,3,3,3-heptafluoropropane
InactiveUS20050222471A1Preparation by dehalogenationPreparation by hydrogen halide split-offCrystalline oxideGas phase
A process for the preparation of 2-chloro-1,1,1,3,3,3-heptafluoropropane is disclosed which involves (a) contacting a mixture comprising hydrogen fluoride, chlorine, and at least one starting material selected from the group consisting of halopropenes of the formula CX3CCl═CX2 and halopropanes of the formula the CX3CClYCX3, wherein each X is independently F or Cl, and Y is H, Cl or F (provided that the number of X and Y which are F totals no more than six) with a chlorofluorination catalyst in a reaction zone to produce a product mixture comprising CF3CClFCF3, HCl, HF, and underfluorinated halogenated hydrocarbon intermediates. The process is characterized by said chlorofluorination catalyst comprising at least one chromium-containing component selected from (i) a crystalline alpha-chromium oxide where at least 0.05 atom % of the chromium atoms in the alpha-chromium oxide lattice are replaced by nickel, trivalent cobalt or both nickel and trivalent cobalt, provided that no more than 2 atom % of the chromium atoms in the alpha-chromium oxide lattice are replaced by nickel and that the total amount of chromium atoms in the alpha-chromium oxide lattice that are replaced by nickel and trivalent cobalt is no more than 6 atom %, and (ii) a fluorinated crystalline oxide of (i). Also disclosed is a process for the manufacture of a mixture of HFC-227ea and hexafluoropropene by reacting a starting mixture comprising CFC-217ba and hydrogen in the vapor phase at an elevated temperature, optionally in the presence of a hydrogenation catalyst. This process involves preparing the CFC-217ba by the process described above.
Owner:EI DU PONT DE NEMOURS & CO
Preparation method of sodium valproate
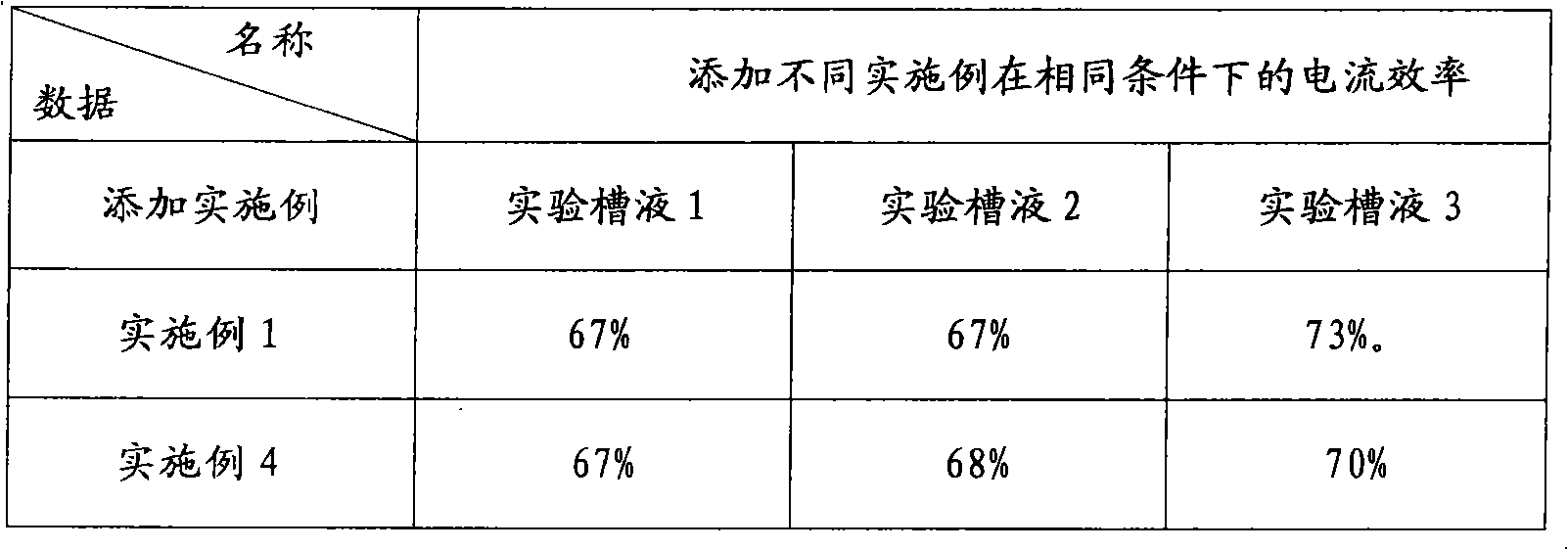
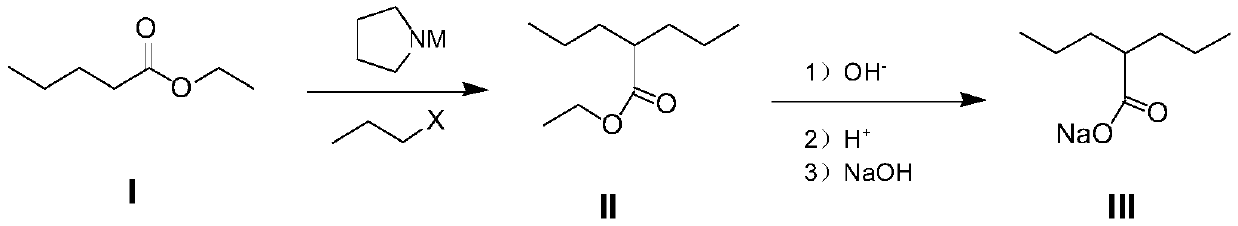
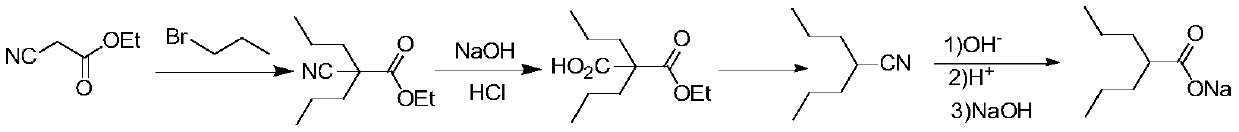
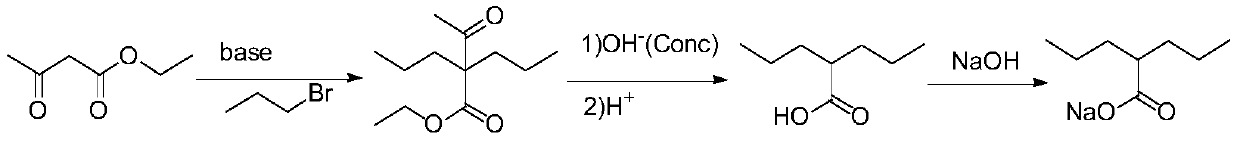
PendingCN111349003AShort reaction pathHigh yieldOxygen-containing compound preparationOrganic compound preparationHalopropanePyrrole
The invention provides a preparation method of sodium valproate, and belongs to the technical field of medicine synthesis. The method comprises the following steps: by taking ethyl valerate as a raw material, adding a methyl tert-butyl ether solution of a pyrrole metal reagent into an ether solution of ethyl valerate, then adding halopropane, carrying out an alkylation reaction, adding a weakly acidic solution in a dropwise manner to terminate the reaction after the reaction is finished, and washing with water to obtain an intermediate product; and adding a sodium hydroxide solution into the alcohol solvent of the intermediate product, carrying out a saponification reaction, and purifying to obtain sodium valproate after the saponification reaction is finished. The method is short in reaction route, high in total yield, easily available in raw materials, low in cost, high in operability and suitable for industrialization. The total molar yield of sodium valproate prepared by the methodis greater than or equal to 86.0%, and the purity of the final product is greater than or equal to 99.5%.
Owner:SICHUAN CREDIT PHARMA
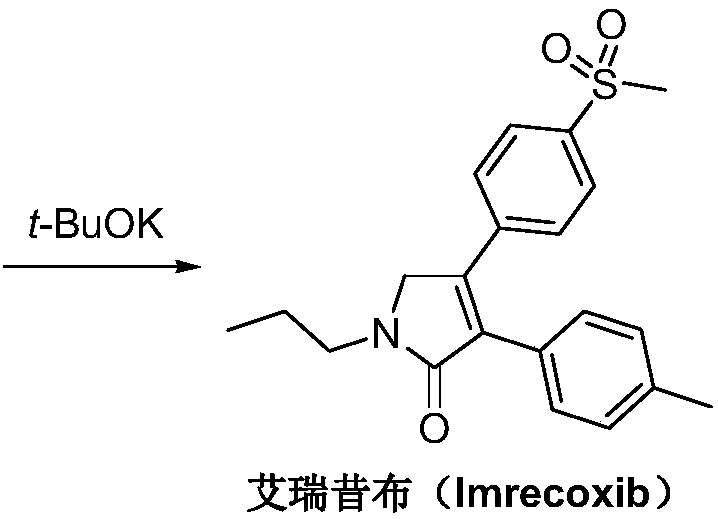
Synthetic method of imrecoxib
The invention discloses a synthetic method of imrecoxib. The synthetic method comprises the following steps: allowing (E)-1-p-methanesulfonylphenyl-1-nitro-2-p-tolylethylene and ethyl isocyanoacetateto undergo a condensation and cyclization reaction in a system containing an alkali reagent and a solvent; allowing the obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylate to undergo a reducing reaction at -78 DEG C in a system containing a reducing reagent and a solvent; allowing the obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carbaldehyde to undergo an oxidation reaction in a system containing a hydrogen peroxide solution and a solvent; and allowing the obtained 3-p-methylphenyl-4-p-methylsulfonylphenyl-3-pyrrolidin-2-one and 1-halopropane or a hydrocarbonpropyl sulfonate derivative to undergo a substitution reaction in a system containing an alkali reagent and a solvent to obtain the imrecoxib. The synthetic method of the imrecoxib has the beneficialeffects that the reaction steps are simplified and optimized and the process is simple in operation, thereby being helpful to reduce cost; reaction impurities are less and controllable, so greennessand environment-friendliness are embodied; and initial raw materials and the used reagents are easily available.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Acid-modified melamine resin for UV (ultraviolet) paint and preparation method thereof
The invention discloses an acid-modified melamine resin for a UV (ultraviolet) paint, which is composed of the following components in parts by weight: 6-13 parts of melamine, 20-50 parts of photocuring agent, 0.01-1 part of polymerization inhibitor, 0.01-1 part of catalyst, 2-5 parts of organic solvent and 20-60 parts of R-epoxy halopropane. The acid-modified melamine resin is used for a UV paint, and can effectively enhance the wear resistance, hardness and heat resistance of the UV paint; and thus, the wear value of the UV paint can reach 0.03g / 100r, the hardness can reach 4H, and the heat resistance can reach 360 DEG C.
Owner:湖南罗比特新材料有限公司
A kind of synthetic method of Erecoxib
ActiveCN108912030BMeet the needs of useSimplify and optimize reaction stepsOrganic chemistryPyrrolidinonesAcyl group
A kind of synthetic method of Erecoxib, step: with (E)-1-p-methylsulfonylphenyl-1-nitro-2-p-tolylethylene and isocyanoacetate in the mixture of alkali reagent and solvent The condensation cyclization reaction is carried out in the system; the obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-carboxylate is reduced at -78°C in a system of reducing agent and solvent Reaction; The obtained 3-p-methylphenyl-4-p-methylsulfonylphenylpyrrole-2-formaldehyde is oxidized in the system of hydrogen peroxide and solvent; the obtained 3-p-methylphenyl-4- Perform substitution reaction between p-methylsulfonylphenyl-3-pyrrolidin-2-one and 1-halopropane or hydrocarbon sulfonate propyl ester derivatives in a system of alkali reagent and solvent to obtain Erecoxib. The reaction steps are simplified and optimized, and the process operation is simple, which helps to reduce the cost; the impurities in the reaction are less and controllable, reflecting green environmental protection; the starting materials and the reagents used are easily available.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Preparation method of epoxy group-containing phthalonitrile monomer
ActiveCN113683585ALow melting pointImprove mechanical propertiesOrganic chemistryEpoxyPolymer science
The invention discloses a preparation method of an epoxy group-containing phthalonitrile monomer, and relates to the technical field of organic synthesis. The method comprises the following steps: blending phenolic hydroxyl group-containing phthalonitrile and epoxy halopropane serving as raw materials in alkali liquor for a reaction under the catalysis of quaternary ammonium salt to obtain a mixed solution of an epoxy group-containing phthalonitrile monomer, and purifying the mixed solution to obtain the epoxy group-containing phthalonitrile monomer. According to the invention, the melting temperature of the phthalonitrile monomer is reduced, and the processing window of phthalonitrile is widened, so the application field of phthalonitrile resin is expanded.
Owner:QILU UNIV OF TECH
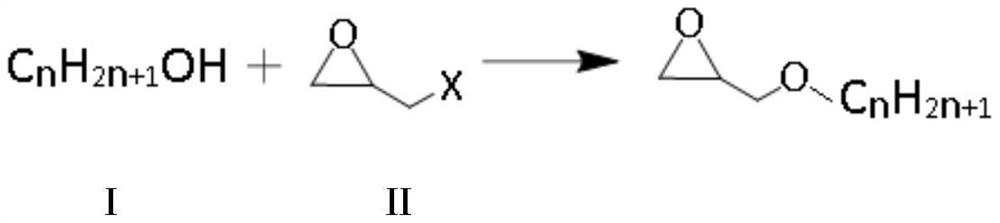
Preparation method of alkyl glycidyl ether
ActiveCN112250646AImprove securityImprove efficiencyOrganic chemistryChemical/physical/physico-chemical microreactorsPolymer scienceAlcohol
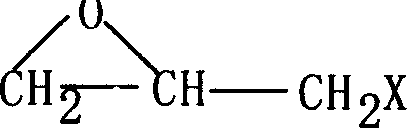
The invention provides a preparation method of alkyl glycidyl ether. The preparation method comprises the following steps: carrying out the following continuous flow substitution reaction on alkyl alcohol and epoxy halogenated propane in a micro-channel reactor to obtain alkyl glycidyl ether; wherein the alkyl alcohol comprises one or a mixture of more of compounds CnH<2n+1>OH shown as a formula I, and n in the formula I is 12-14; the epoxy halopropane has a structure shown as a formula II, and X in the formula II is a halogen atom. The invention solves the problems that in the prior art, whenalkyl glycidyl ether is prepared, reaction safety is poor, the number of byproducts is large, and the product yield and purity cannot be considered at the same time; the preparation method is simplein operation process, high in product purity, efficient, safe, environmentally friendly and suitable for industrial production.
Owner:RIANLON ZHONGWEI NEW MATERIAL CO LTD +1
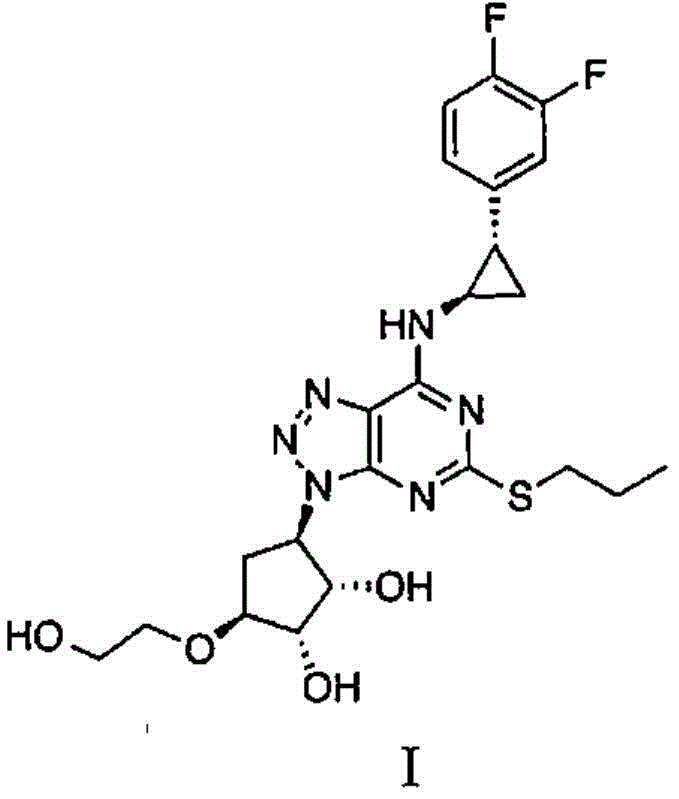
A kind of preparation method of ticagrelor intermediate
Owner:JIANGXI SYNERGY PHARMA
Synthesis method of chiral sec-allyl alcohol with hydroxyl ortho-position replaced with halogen atoms
InactiveCN106588565AHigh optical purityHigh E-stereoselectivityGroup 4/14 element organic compoundsOrganic compound preparationEpoxyTetrazole
The invention discloses a method for synthesizing optically-pure chiral sec-allyl alcohol by means of an improved Julia alkylation reaction, and belongs to the technical field of organic synthesis. The method comprises the specific technological steps that high optically-pure epoxy halopropane is subjected to hydroxyl protection after tetrazole-thione nucleophilic ring opening is performed and oxidized into an improved Julia alkylation reagent to be condensed with aldehyde or ketone, hydroxyl protection is removed, and a series of high optically-pure chiral sec-allyl alcohol containing E-type carbon-carbon double bonds can be obtained.
Owner:JIANGNAN UNIV
Cationic gemini fluorosurfactant based on perfluorononene and perfluorohexene and preparation method thereof
ActiveCN102908937BOrganic compound preparationTransportation and packagingBenzoic acidPolymer science
The invention discloses a cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and a preparation method of the cationic type gemini fluorinated surfactant. The preparation method comprises the following steps of: with the perfluorinated nonene or perfluorinated hexene as a raw material, condensing the perfluorinated nonene or perfluorinated hexene with p-hydroxybenzoic acid, and carrying out chlorination on a matter obtained after condensation and thionyl chloride to prepare perfluorinated alkene oxyl benzoyl chloride; condensing the perfluorinated alkene oxyl benzoyl chloride without separation with 2-dimethylamino ethanol, 3-dimethylamino propanol, 2-dimethylamino ethylamine and 3-dimethylamino propylamine to obtain perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with tertiary amine at an alkanol part or amine part; and finally, condensing the perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with 1,2-dihaloethane, 1,3-dihalopropane, 1,4-dichlorobutane, 1,5-dichloropentane or 3-oxa-1,5-dichloropentane to prepare a quaternary ammonium gemini fluorinated surfactant. The synthesized compound is high in surface activity and is low in critical micelle concentration, has the characteristics of simpleness in synthesization, low cost and the like and has good application prospect.
Owner:江苏超至和新材料有限公司
Reaction type antistatic agent, preparation method and durable antistatic polyurethane material
ActiveCN103113575BGood dissipation performanceIncreased durabilitySolesOrganic compound preparationEpoxyFoaming agent
The invention provides a reaction type antistatic agent. The antistatic agent is generated by reacting an intermediate which is synthesized under the alkaline condition based on polyethylene glycol and epoxy halopropane as raw materials with trialkylamine haloid, wherein the molecular weight of polyethylene glycol is 400-2,000. The invention also provides a preparation method of the reaction type antistatic agent, which comprises the following steps of: (1) synthesizing the intermediate at 40-60 DEG C under the alkaline condition by taking polyethylene glycol and epoxy halopropane as the raw materials; and (2) reacting the intermediate with trialkylamine haloid in an alcohol solvent at 20-40 DEG C to obtain the reaction type antistatic agent. The invention also provides an antistatic polyurethane material which is prepared by mixing and pouring a raw material A and a raw material B, wherein the raw material A comprises polyhydric alcohol, a catalyst, a foaming agent, a chain extender and the reaction type antistatic agent; and the raw material B is isocyanate.
Owner:SHENZHEN SELEN SCI & TECH CO LTD
Preparation method of ticagrelor intermediate
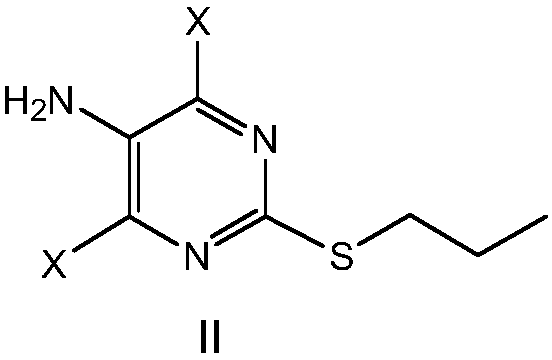
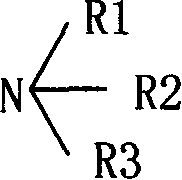
The invention provides a preparation method of 4,6-dihalo-2-(propylthio)-5-aminopyrimidine disclosed as structural formula II, which comprises the following steps: 1) reacting a compound disclosed as structural formula V with 1-propyl hydrosulfide or 1-halopropane in the presence of alkali to obtain 5-halo-2-propylthiopyrimidine disclosed as structural formula IV; 2) reacting the 5-halo-2-propylthiopyrimidine disclosed as structural formula IV with ammonia gas, ammonia water or ammonium salt in the presence or absence of alkali to obtain 5-amino-2-propylthiopyrimidine disclosed as structural formula III; and 3) reacting the 5-amino-2-propylthiopyrimidine disclosed as structural formula III with a halogenating reagent to obtain the 4,6-dihalo-2-(propylthio)-5-aminopyrimidine disclosed as structural formula II. The preparation method has the advantages of short reaction route, high product purity and cheap and accessible raw materials, and is suitable for industrialized mass production. In the formulae, Q and X are respectively and independently selected from chlorine, bromine or iodine; and Y is OH or SH.
Owner:JIANGXI SYNERGY PHARMA
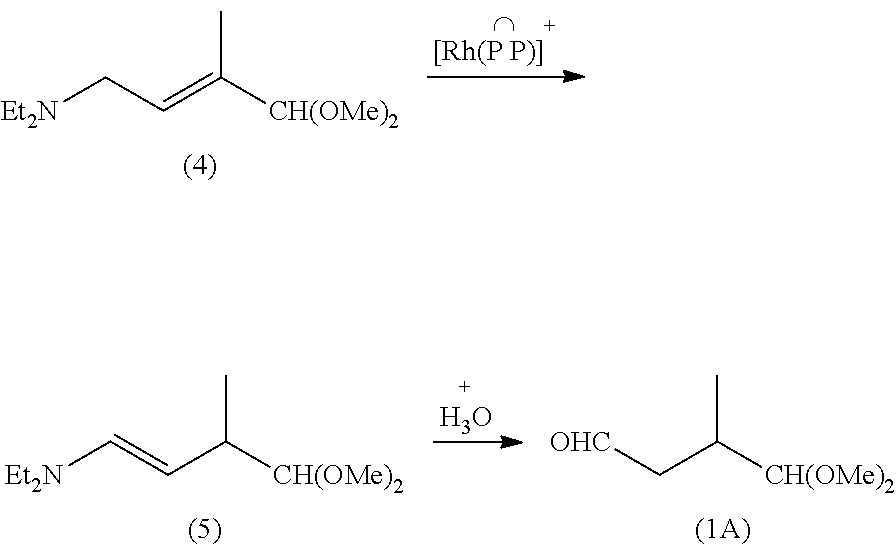
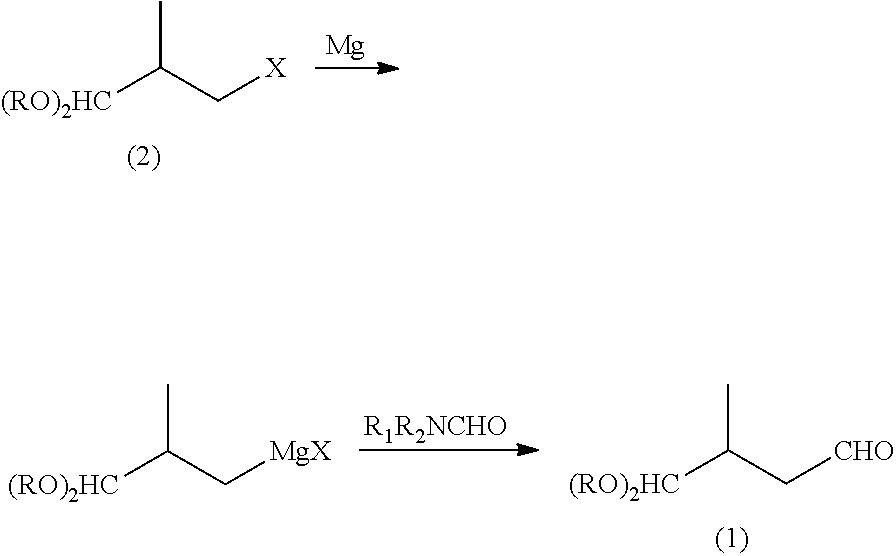
Preparation method of lycopene intermediate 3-methyl-4,4-dialkoxy-1-butaldehyde
ActiveUS8993810B2Readily availableSimple process routeOrganic compound preparationCarbonyl compound preparationGrignard reagentLycopene
Disclosed is a preparation method of the lycopene intermediate 3-methyl-4,4-dialkoxy-1-butaldehyde. The preparation method comprises the following steps: (1) reacting 2-methyl-3,3-dialkoxy-1-halopropane with magnesium powder in the solvent of anhydrous tetrahydrofuran at a temperature of 45˜65° C. to generate a mixture of Grignard reagents under the protection of an inert gas; and (2) adding N,N-disubstituted carboxamide to the mixture of Grignard reagents and reacting at a temperature of 10° C.˜35° C. to obtain 3-methyl-4,4-dialkoxy-1-butaldehyde. The process route of the present invention is simple and direct, the operation is easy, the conditions are mild and the yield is good, and thus the invention has commercial value.
Owner:NANJING TECH UNIV +1
A kind of preparation method of Erecoxib intermediate and Erecoxib
ActiveCN108707100BReduce usageMeet high standardsOrganic chemistryOrganic compound preparationEthyl groupAcyl group
Owner:江苏美迪克化学品有限公司
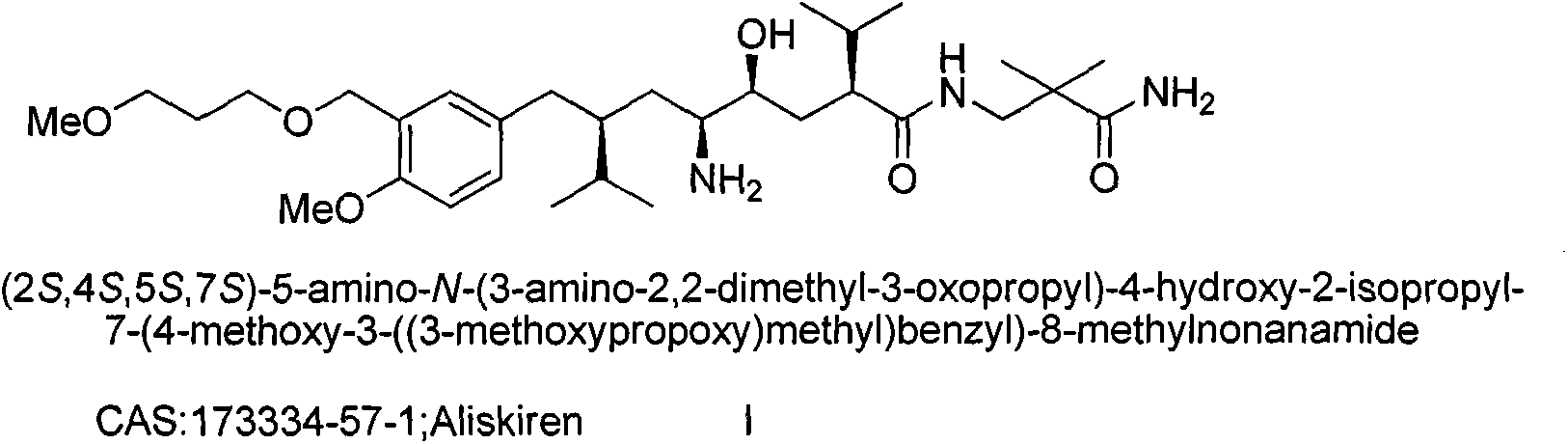
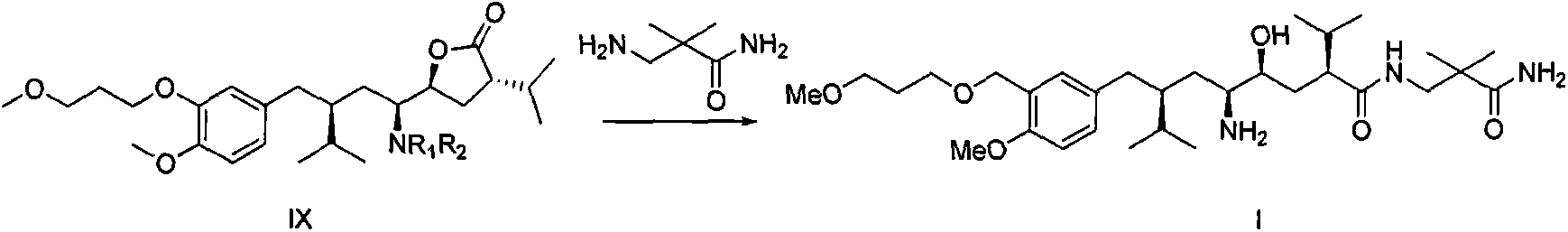
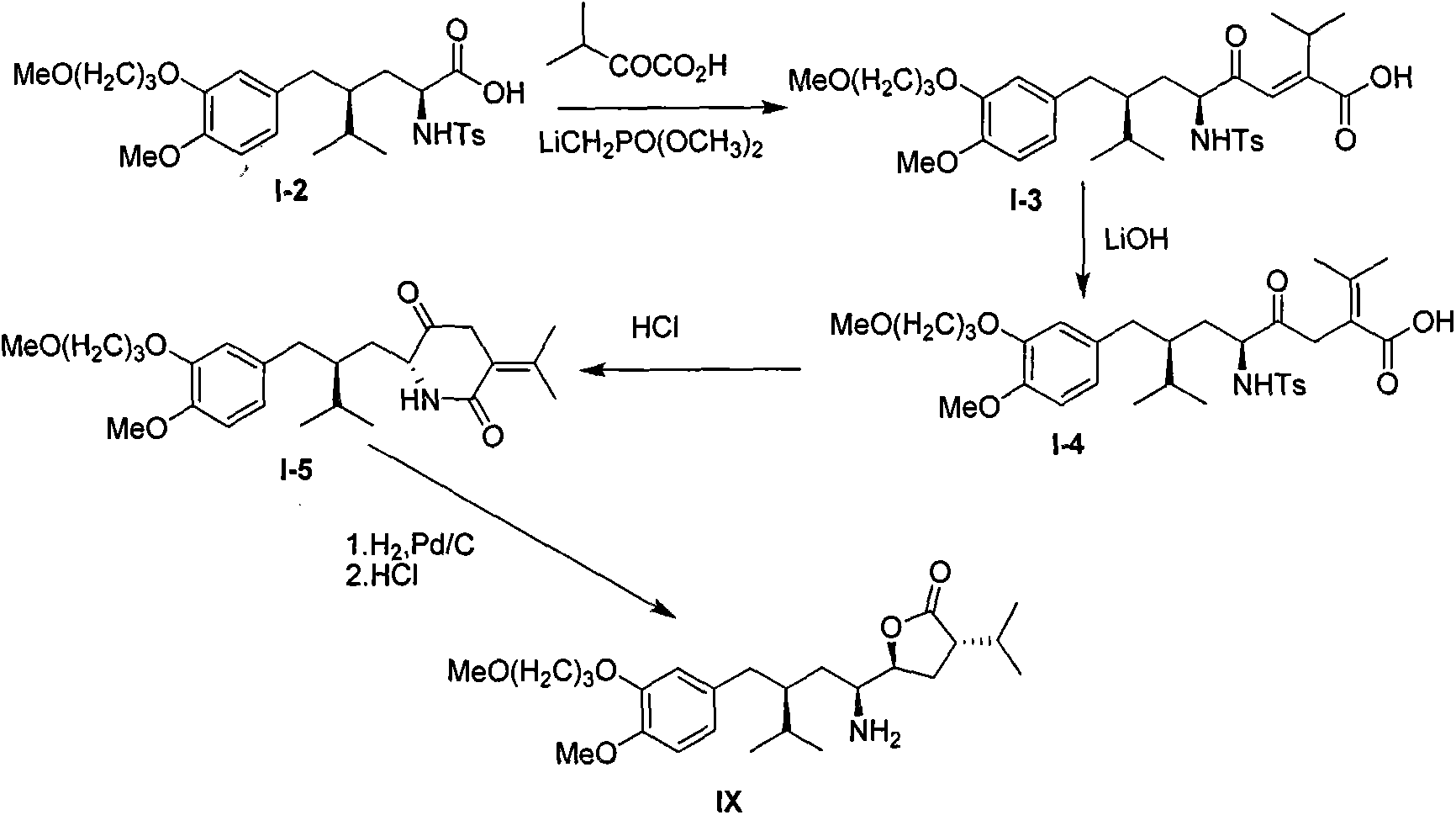
Preparation method for intermediate 3-isopropylfuranone derivatives of blood-pressure-reducing medicine aliskiren
InactiveCN103980234ARaw materials are easy to getEasy to operateOrganic chemistryBulk chemical productionBromochloromethaneBromine
The invention relates to a preparation method for intermediates 3-isopropylfuranone derivatives of a blood-pressure-reducing medicine aliskiren, and belongs to the technical field of medicine preparation methods. The method is characterized by comprising: taking (2S,4S)-2-amino-4-(4-methoxy-3-(3-methoxypropoxy)benzyl)-5-methylcaproic acid (compound II) as a raw material, and performing amino protection to obtain a compound III; reacting the compound III with bromochloromethane to obtain a compound IV; performing reduction and ring-closing reaction on the compound IV to obtain a compound VI; reacting the compound VI with diethyl malonate to obtain a compound VII; reacting the compound VII with a 2-halopropane to obtain a compound VIII; and performing decarboxylation on the compound VIII to obtain a compound IX. The method has the advantages of raw material easy availability, operation simpleness, method reliability, low cost, easy industrialization and the like.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Gemini-type ampholytic surfactant based on perfluoroolefine and preparation method thereof
InactiveCN103657513BImprove surface activityGood dispersionTransportation and packagingMixingActive agentPropylamine
The invention discloses a Gemini-type ampholytic surfactant based on perfluorononene and perfluorohexene, and a preparation method thereof. The preparation method comprises the following steps: taking perfluorononene and perfluorohexene as the raw materials, carrying out phenol condensation reactions and chlorosulfonation reactions so as to obtain perfluoroolefineoxyl benzene sulfonyl chloride, then subjecting the perfluoroolefineoxyl benzene sulfonyl chloride to carry out condensation reactions with 2-dimethylamino ethylamine or 3-dimethylamino propylanmine so as to obtain perfluoroolefineoxyl benzene sulfonamide whose amine part comprises a tertiary amine group, then subjecting the perfluoroolefineoxyl benzene sulfonamide to carry out condensation reactions with 1,2-dihaloethane, 1,3-dihalopropane, 1,4-dihalobutane, 1,5-dihalopentane, 3-oxa-1,5-dihalopentane, or 1,3-dihaloacetone, wherein the halogen element is chlorine or bromine, and finally subjecting the reaction product to carry out condensation reactions with vinyl sulfonate, chloroethyl sulfonate, bromoethyl sulfonate or 1,3-propanesultone so as to obtain the Gemini-type ampholytic fluorine surfactant with excellent surface activity and low critical micelle concentration. The Gemini-type ampholytic fluorine surfactant has the prominent characteristics of simple synthetic operation, low cost, high surface activity, and low using concentration, and has a vast market prospect.
Owner:QUZHOU UNIV
Acid-modified melamine resin for uv coating and preparation method thereof
The invention discloses an acid-modified melamine resin for a UV (ultraviolet) paint, which is composed of the following components in parts by weight: 6-13 parts of melamine, 20-50 parts of photocuring agent, 0.01-1 part of polymerization inhibitor, 0.01-1 part of catalyst, 2-5 parts of organic solvent and 20-60 parts of R-epoxy halopropane. The acid-modified melamine resin is used for a UV paint, and can effectively enhance the wear resistance, hardness and heat resistance of the UV paint; and thus, the wear value of the UV paint can reach 0.03g / 100r, the hardness can reach 4H, and the heat resistance can reach 360 DEG C.
Owner:湖南罗比特新材料有限公司
Method for producing 2,3,3,3-tetrafluoropropene
ActiveUS9346723B2Overcomes drawbackSimple designSolidificationLiquefactionHydrogen fluorideDistillation
Owner:ARKEMA FRANCE SA
Polishing agent for alkaline non-cyanide zincate zinc plating and process for preparing polishing agent composition
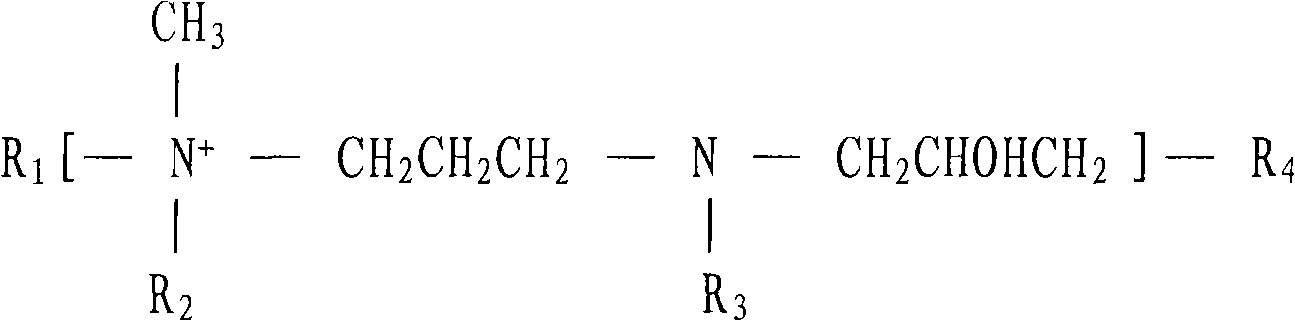
The basic cyanide-free zincate galvanizing brightener and its composition brightener are copolymerized polymers produced by random copolymerization of compounds in the following molar ratios, the compounds and their molar ratios being: at least one nitrogen-containing Cyclic compound: at least one hydroxylamine: at least one di-tertiary amine containing acyl or sulfonyl group: epihalohydrin and / or glycerol halohydrin=0.5~5.0: 0.5~4.0: 0.5~5.0: 4.0~10.0; The preparation method is as follows: heating the nitrogen-containing heterocyclic compound, hydroxylamine, di-tertiary amine containing acyl group or sulfonyl group and an appropriate amount of water in the above formula, and adding epihalohydrin and / or glycerin halohydrin while stirring , the temperature is controlled below the reflux temperature, the time is 0.5-2 hours, then the temperature is raised to the reflux temperature, and the temperature is kept for 4-10 hours; the invention is easily soluble in water and alkaline solution, and is applied in the alkaline cyanide-free galvanizing process , The range of current density is wide, the total current of the Hull cell is 3A, the high current area does not burn, and the coating with excellent physical properties and bright mirror surface can be obtained, and the cost is low.
Owner:JIANGMEN DESHANG KEZUO TECHNOLOGY INDUSTRIAL CO LTD
Preparation method of polysulfate type epoxy resin
ActiveCN113929871AGood acid and alkali resistanceSolve the disadvantage of lower thermal performanceEpoxyPolymer science
The invention relates to a preparation method of polysulfate type epoxy resin. The preparation method comprises the steps of (1) preparing polysulfate of which the number-average molecular weight is 1000-10000 g / mol and the terminal group is hydroxyl; (2) heating and dissolving polysulfate in epoxy halopropane; (3) continuing to add a ring-opening catalyst, and enabling epoxy halopropane to react with polysulfate; (4) continuing to add a ring-closed catalyst, and enabling the end group of the polysulfate to be changed into an epoxy structure; and (5) separating out the product, and purifying. The preparation method provided by the invention can realize large-scale industrial production, and has the advantages of simple post-treatment process and small pollution.
Owner:BAIYIN TUWEI NEW MATERIALS TECHNOLOGY CO LTD
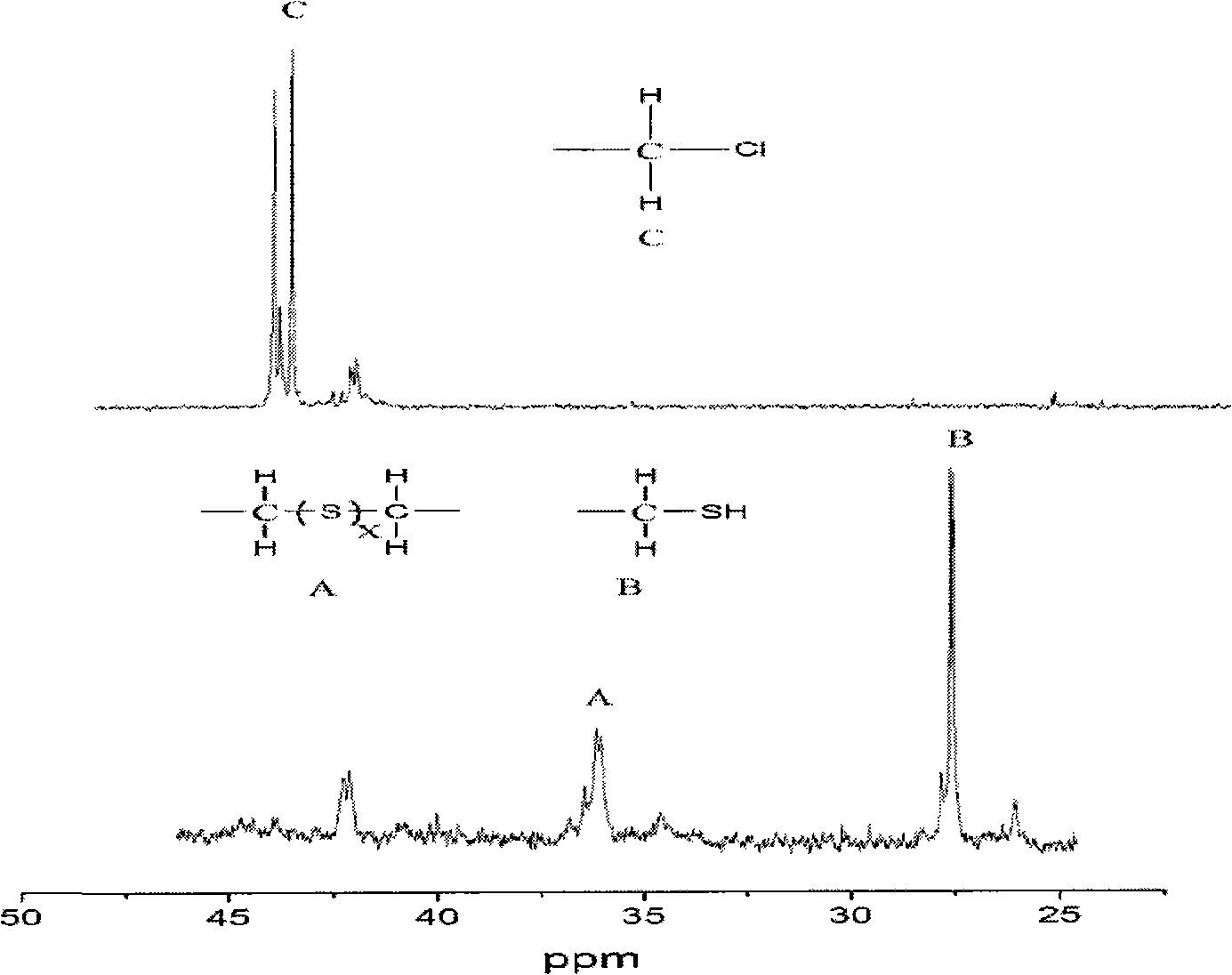
Method for preparing mercapto polyether
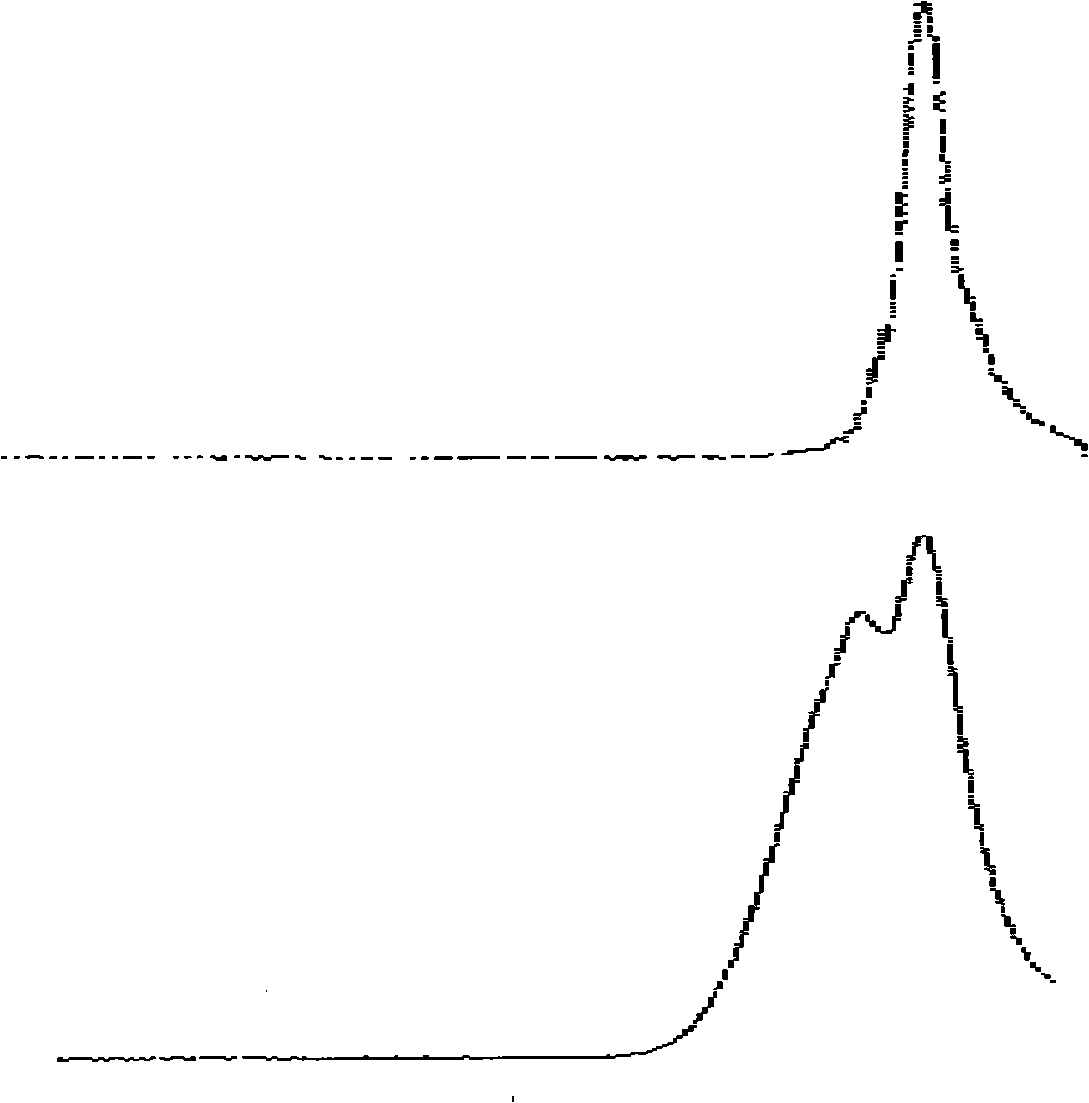
The invention relates to a preparation method of end-mercapto polyether, belonging to adhesive field. The deficits of the present preparation method of end-mercapto polyether: the solubleness of product obtained by reacting polyalkylene diol and epoxy halopropane in sodium hydrosulphide is poor and the availability of sodium hydrosulphide is reduced, therefore the difficulty is added to the post-treatment. The chloride polyether, sodium hydrosulphide and alcohol material are mixed together and react for 2-5 hours at boiling point of alcohol material to 40 degree, wherein the quantity relativeratio of alcohol material and sodium hydrosulphide is 3-18:1, the quantity relative ratio of chloride polyether and sodium hydrosulphide is 1:2-3.5. The availability of the sodium hydrosulphide is greatly increased. In addition, the displacement reaction of chloride polyether and sodium hydrosulphide occurs in alcohol material, the reaction temperature is reduced, so as to reduce the energy consumption.
Owner:BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com