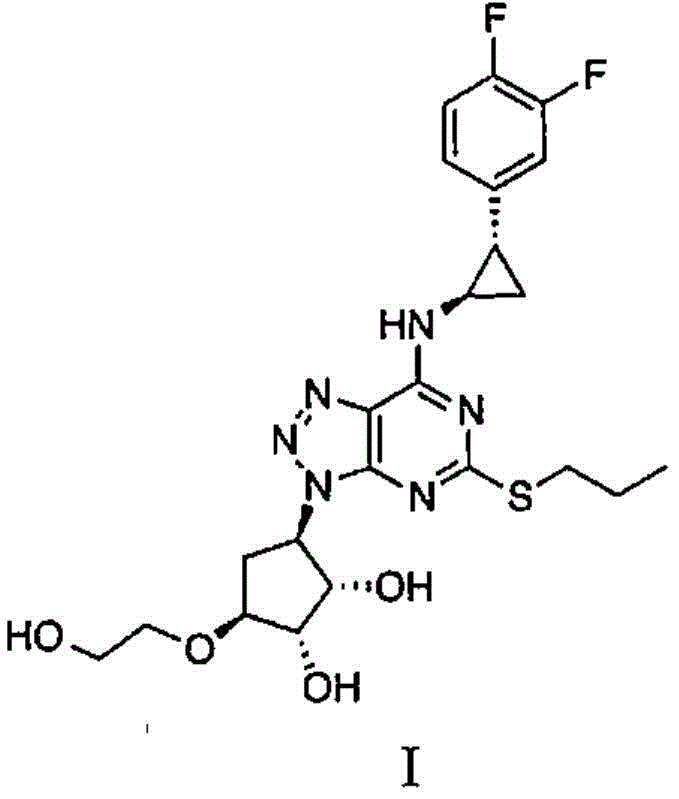
Preparation method of ticagrelor intermediate
A structural formula and selected technology, applied in the direction of organic chemistry, can solve the problems of impact, long reaction steps, unfavorable industrial production, etc., and achieve the effect of cheap and easy-to-obtain raw materials, short reaction steps, large cost and benefit advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
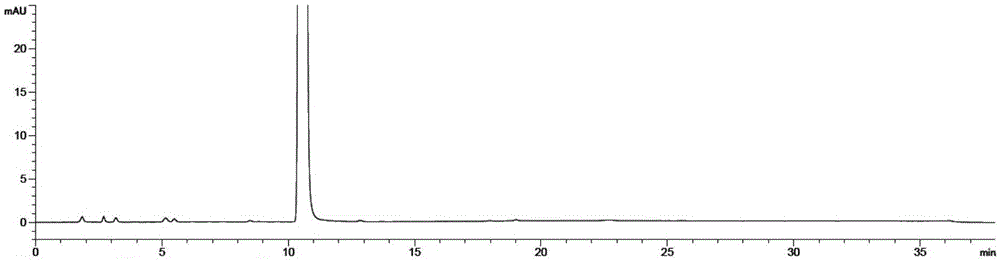
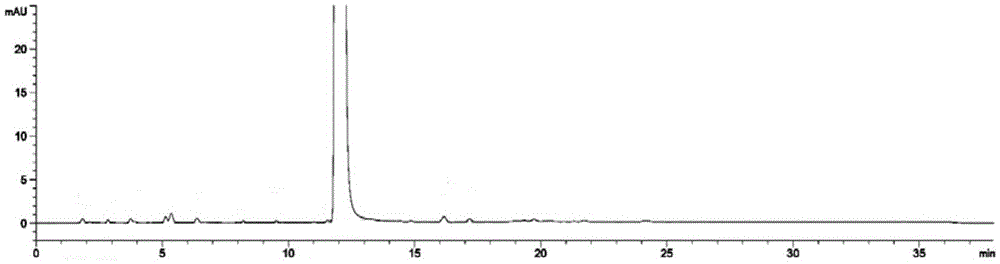
Image
Examples
Embodiment 14
[0067] The preparation of embodiment 14,6-dibromo-2-(propylthio)-5-aminopyrimidine
[0068] 1) Preparation of 5-bromo-2-propylthiopyrimidine
[0069] Put 50g of 5-bromo-2-hydroxypyrimidine (0.286mol), 250ml of dichloromethane, 80g of potassium carbonate (0.58mol) into the reaction flask and stir for 20min, drop in 30g of 1-propanethiol (0.395mol), and stir at 40°C React for 6 hours, put 100ml of water into the reaction bottle, stir for 30 minutes, let stand, separate layers, extract the water layer with dichloromethane 1 to 2 times, combine the organic layers, wash once with water, and evaporate the organic layer to dryness under reduced pressure, leaving Add 90ml of methanol to the mixture, heat up to 60°C to dissolve, slowly cool down to 0°C, keep stirring for 2 hours, filter, and dry the filter cake to obtain 5-bromo-2-propylthiopyrimidine (56.6g, yield 85%).
[0070] 1 HNMR (400MHz, CDCl 3 ):8.51(s,2H),2.95(m,2H),1.65(m,2H),0.95(m,3H).
[0071] 2) Preparation of 5-amin...
Embodiment 2
[0092] Example 2 Preparation of 4,6-dichloro-2-(propylthio)-5-aminopyrimidine
[0093] 1) Preparation of 5-chloro-2-propylthiopyrimidine
[0094] Put 50g (0.341mol) of 5-chloro-2-thiopyrimidine in the reaction bottle, 80g triethylamine (0.79mol), 250ml ethyl acetate, drop into 22.6g1-chloropropane (0.5mol) under stirring, in 40 Stir at °C for 7 hours. Put 100ml of water into the reaction bottle, let it stand, separate the organic layer and the water layer; the separated water layer was extracted twice with ethyl acetate, the combined organic layer was washed twice with water, the organic layer was evaporated to dryness under reduced pressure, and the residue was Add 100ml of methanol, heat up to 60°C to dissolve, slowly cool down to 0°C, keep stirring for 2 hours, filter, and dry the filter cake to obtain 5-chloro-2-propylthiopyrimidine (56g, yield 87%).
[0095] 1 HNMR (400MHz, CDCl 3 ):8.49(s,2H),2.93(m,2H),1.64(m,2H),0.94(m,3H)
[0096] 2) Preparation of 5-amino-2-pr...
Embodiment 3
[0103] Example 3 4,6-Dichloro-2-(propylthio)-5-aminopyrimidine
[0104] 1) Preparation of 5-bromo-2-propylthiopyrimidine
[0105] Put 50g of 5-bromo-2-hydroxypyrimidine (0.383mol), 250ml of toluene, 23g of sodium hydroxide (0.575mol) into the reaction flask and stir for 20min, then add 58g of 1-propanethiol (0.763mol) dropwise, and stir at 10°C 10 hours. Put 100ml of water into the reaction bottle, let it stand, separate the organic layer and the water layer; extract the separated water layer with toluene 1 to 2 times, combine the toluene and the organic layer, wash with water once, and evaporate the organic layer to dryness under reduced pressure, leaving Throw in 90ml of methanol, heat up to 60°C to dissolve, slowly cool down to 0°C, keep stirring for 2h, filter, and dry the filter cake to obtain 5-bromo-2-propylthiopyrimidine (77g, yield 86.5%).
[0106] 1 HNMR (400MHz, CDCl 3 ):8.51(s,2H),2.95(m,2H),1.65(m,2H),0.95(m,3H)
[0107] 2) Preparation of 5-amino-2-propylth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com