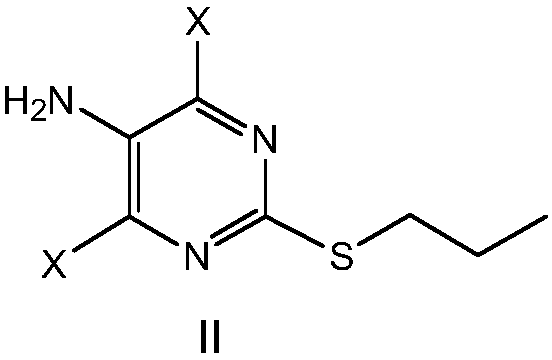
A kind of preparation method of ticagrelor intermediate
A structural formula, selected technology, applied in the direction of organic chemistry, etc., can solve the problems of influence, long reaction steps, unfavorable industrial production, etc., and achieve the effects of cheap and easy-to-obtain raw materials, short reaction steps, high cost and benefit advantages.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
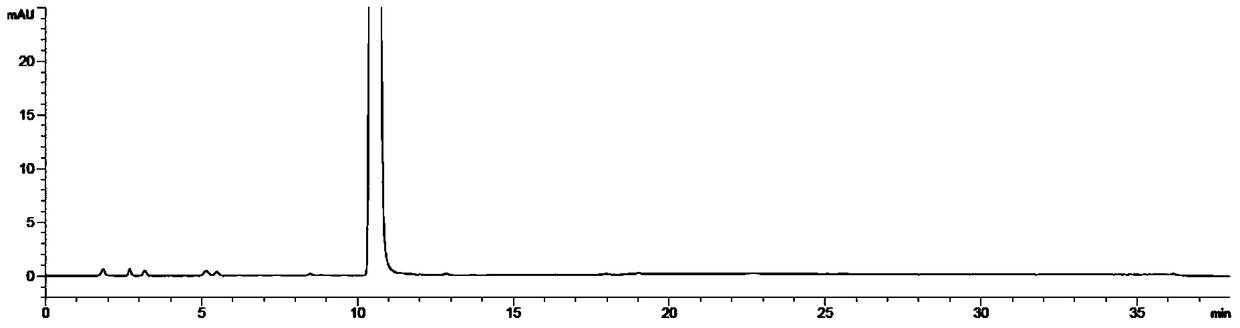
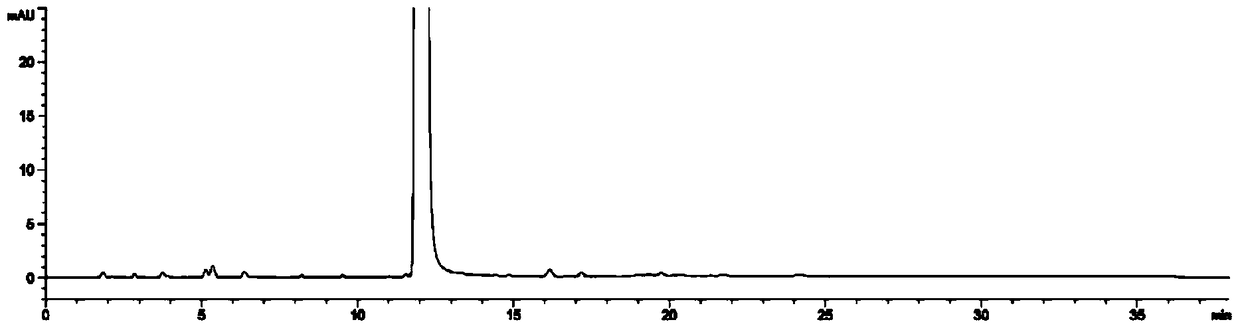
Image
Examples
Embodiment 1
[0067] Example 1 Preparation of 4,6-dibromo-2-(propylthio)-5-aminopyrimidine
[0068] 1) Preparation of 5-bromo-2-propylthiopyrimidine
[0069] Put 50g 5-bromo-2-hydroxypyrimidine (0.286mol), 250ml dichloromethane, 80g potassium carbonate (0.58mol) into the reaction flask, stir for 20min, drop in 1-propanethiol 30g (0.395mol), at 40℃ Stir the reaction for 6 hours, put 100ml of water into the reaction flask, stir for 30 minutes, stand still, separate the layers, extract the water layer with dichloromethane for 1 to 2 times, combine the organic layers, wash with water once, and evaporate the organic layer under reduced pressure. Add 90 ml of methanol to the residue, warm up to 60°C to dissolve, slowly lower the temperature to 0°C, keep stirring for 2h, filter, and dry the filter cake to obtain 5-bromo-2-propylthiopyrimidine (56.6g, yield 85%).
[0070] 1 HNMR(400MHz, CDCl 3 ): 8.51 (s, 2H), 2.95 (m, 2H), 1.65 (m, 2H), 0.95 (m, 3H).
[0071] 2) Preparation of 5-amino-2-propylthiopyrimid...
Embodiment 2
[0093] Example 2 Preparation of 4,6-dichloro-2-(propylthio)-5-aminopyrimidine
[0094] 1) Preparation of 5-chloro-2-propylthiopyrimidine
[0095] Put 50g (0.341mol) of 5-chloro-2-thiopyrimidine, 80g of triethylamine (0.79mol), and 250ml of ethyl acetate into the reaction flask, and add 22.6g of 1-chloropropane (0.5mol) dropwise with stirring. Stir at 40°C for 7 hours. Put 100ml of water into the reaction flask, let it stand, and separate the organic layer and the water layer; the separated aqueous layer was extracted twice with ethyl acetate, the organic layers were combined, washed twice with water, the organic layer was evaporated to dryness under reduced pressure, and the residue Put in 100ml of methanol, raise the temperature to 60°C to dissolve, slowly lower the temperature to 0°C, keep stirring for 2h, filter, and dry the filter cake to obtain 5-chloro-2-propylthiopyrimidine (56g, yield 87%).
[0096] 1 HNMR(400MHz, CDCl 3 ): 8.49 (s, 2H), 2.93 (m, 2H), 1.64 (m, 2H), 0.94 (...
Embodiment 3
[0104] Example 3 4,6-Dichloro-2-(propylthio)-5-aminopyrimidine
[0105] 1) Preparation of 5-bromo-2-propylthiopyrimidine
[0106] Put 50g 5-bromo-2-hydroxypyrimidine (0.383mol), 250ml of toluene, 23g sodium hydroxide (0.575mol) into the reaction flask and stir for 20min. Add 58g (0.763mol) of 1-propanethiol dropwise at 10℃ Stir for 10 hours. Put 100ml of water into the reaction flask, let it stand, and separate the organic layer and the aqueous layer; the separated aqueous layer is extracted with toluene for 1 to 2 times, the toluene and the organic layer are combined, washed once with water, and the organic layer is evaporated to dryness under reduced pressure. The substance was put into 90ml methanol, heated to 60°C to dissolve, slowly cooled to 0°C, kept stirring for 2h, filtered, and the filter cake was dried to obtain 5-bromo-2-propylthiopyrimidine (77g, yield 86.5%).
[0107] 1 HNMR(400MHz, CDCl 3 ): 8.51 (s, 2H), 2.95 (m, 2H), 1.65 (m, 2H), 0.95 (m, 3H)
[0108] 2) Preparat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com