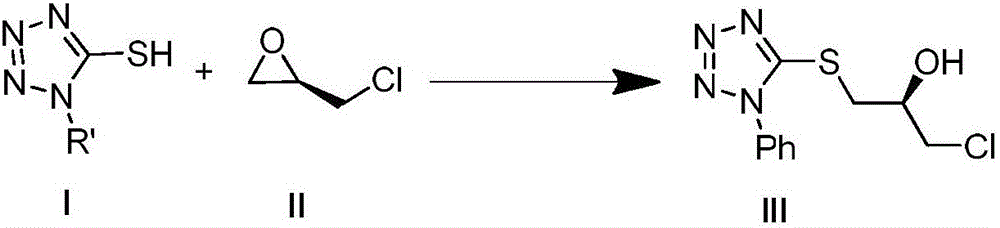Synthesis method of chiral sec-allyl alcohol with hydroxyl ortho-position replaced with halogen atoms
A technology for the synthesis of secondary allyl alcohol and its synthesis method, which is applied in the field of synthesis of chiral secondary allyl alcohol, can solve the problems of low yield, high difficulty in separation and purification, and low stereoselectivity of E-form, and achieve high optical purity and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The embodiments of the present invention are described in detail below: the present embodiment is implemented under the premise of the technical solution of the present invention, and detailed implementation methods and specific operating procedures are provided, but the protection scope of the present invention is not limited to the following implementation example.
[0030] 1. The synthetic reaction formula of (R)-1-chloro-3-(1-phenyl-5-mercaptotetrazole)-2-propanol:
[0031]
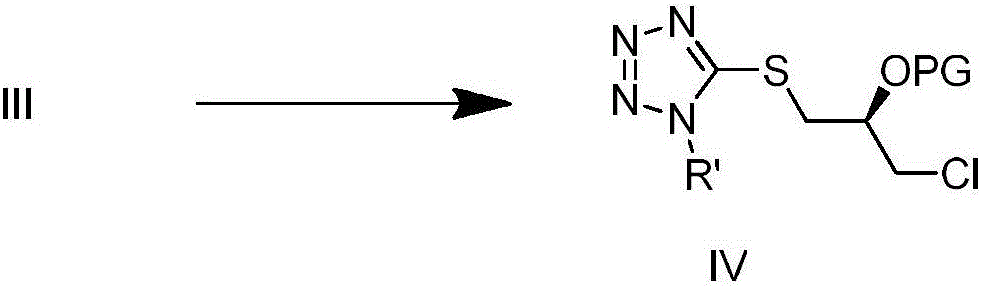
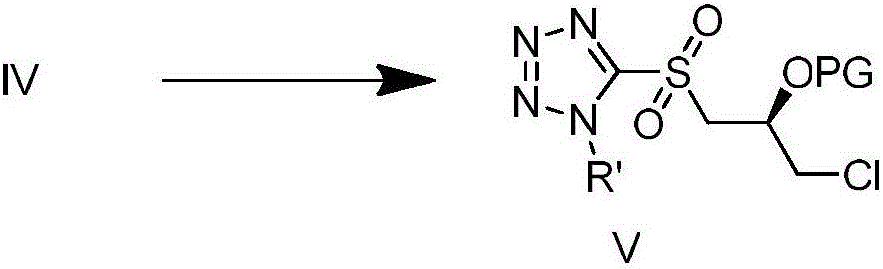
[0032] Steps
[0033] Add 1-phenyl-5-mercaptotetrazolium (50g, 280.6mmol), methanol (350mL), epichlorohydrin (23mL, 294.6mmol) and triethylamine (59mL, 420.8mmol) into a 1000mL round bottom flask , and the reaction was stirred overnight at room temperature. Concentrate the reaction solution, add 150mL ethyl acetate, water, 0.1M hydrochloric acid, saturated NaHCO 3 , washed with saturated brine, separated, and the organic phase was dried over anhydrous magnesium sulfate, filtered, and conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com