Method for preparing mercapto polyether
A technology of mercapto polyether and chlorinated polyether, which is applied in the field of preparation of mercapto-terminated polyether, can solve the problems of poor dispersion of sodium hydrosulfide and low utilization rate of sodium hydrosulfide, etc., to reduce energy consumption, improve utilization rate, and react The effect of temperature reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of mercapto-terminated polyether:
[0024] Raw materials: chlorinated polyether, the number-average molecular mass Mn=1277, and a functionality of 3; sodium hydrosulfide, with a purity of 70%; ethanol as the solvent.
[0025] Add 250g of chlorinated polyether and 46.99g of sodium hydrosulfide into 200ml of ethanol solvent, heat up to 78°C under stirring, and stop the reaction after reacting at this temperature for 2 hours, remove the salt from the reactant, and distill to obtain a transparent, light yellow liquid Polymer terminated mercapto polyether.
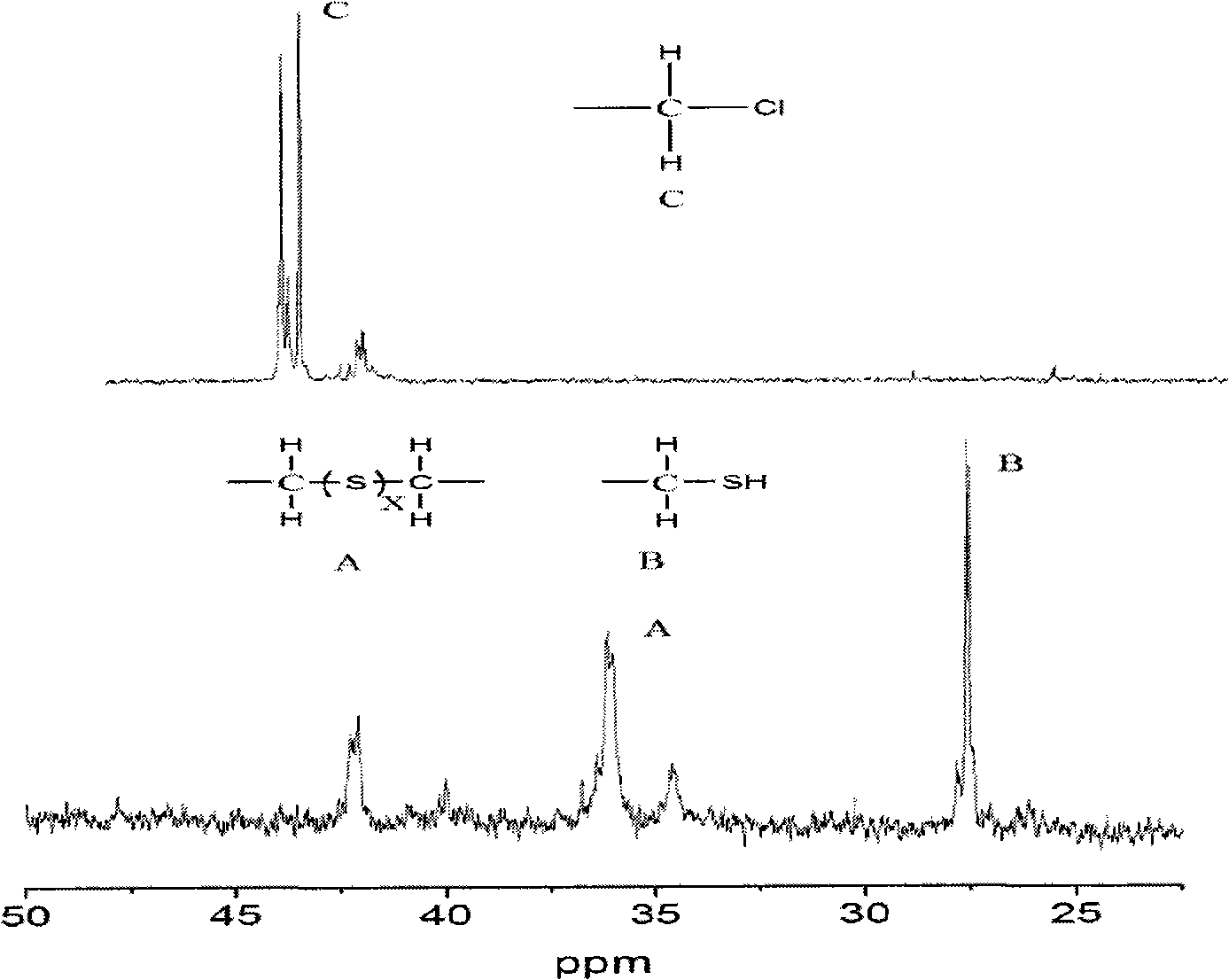
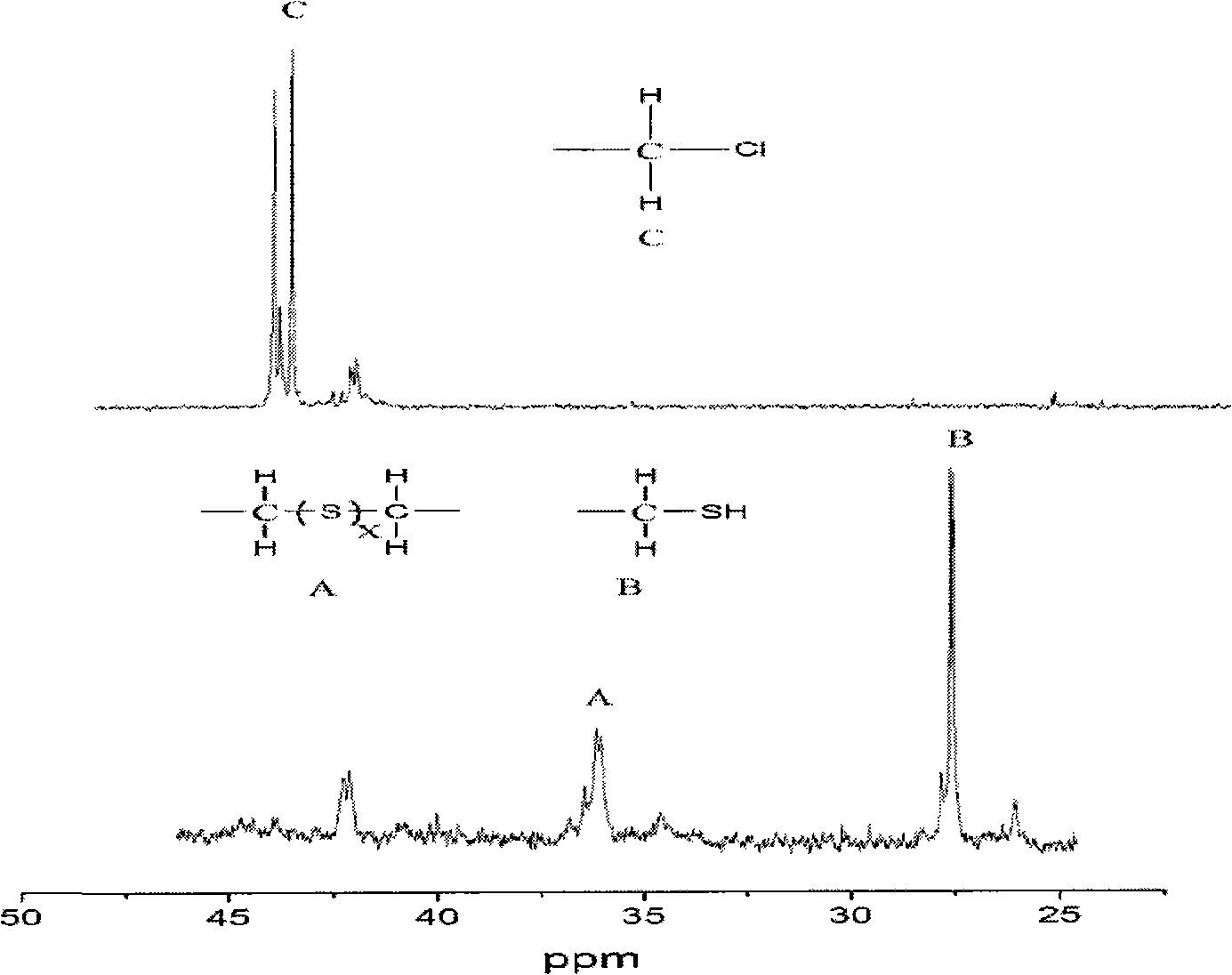
[0026] The synthetic product is liquid at room temperature, and it is 13 C NMR characterization, compared with the NMR spectrum of chlorinated polyether, the characteristic peak at 44.0-45.0ppm disappeared, indicating that the C-CI group was reacted; new characteristic peaks appeared at 25.5ppm and 35.2ppm, indicating that There are C-SH and C-Sx-C (X is 1-5) groups generated. The GPC characterization of t...
Embodiment 2
[0029] Preparation of mercapto-terminated polyether:
[0030] Raw materials: chlorinated polyether, the number-average molecular mass Mn=1277, and a functionality of 3; sodium hydrosulfide, with a purity of 70%; and isopropanol as the solvent.
[0031] Add 250g of chlorinated polyether and 46.99g of sodium hydrosulfide into 250ml of isopropanol solvent, raise the temperature to 82°C under stirring, and stop the reaction after reacting at this temperature for 3 hours, desalinate the reactant, and distill to obtain transparent, shallow Yellow liquid polymer mercapto-terminated polyether.
[0032] The synthetic product is liquid at room temperature, and it is 13 C NMR characterization, compared with the NMR spectrum of chlorinated polyether, the characteristic peak at 44.0-45.0ppm disappeared, indicating that the C-CI group was reacted; new characteristic peaks appeared at 25.5ppm and 35.2ppm, indicating that There are C-SH and C-Sx-C (X is 1-5) groups generated. The GPC chara...
Embodiment 3
[0035] Preparation of mercapto-terminated polyether:
[0036] Raw materials: chlorinated polyether, number average relative molecular mass Mn=1277, functionality 3; sodium hydrosulfide, purity 70%; tert-butanol as solvent.
[0037] Add 250g of chlorinated polyether and 46.99g of sodium hydrosulfide into 300ml of tert-butanol solvent, raise the temperature to 82°C under stirring, and stop the reaction after reacting at this temperature for 3.5 hours, desalt the reactant, and distill it to obtain transparent, shallow Yellow liquid polymer mercapto-terminated polyether.
[0038] The synthetic product is liquid at room temperature, and it is 13 C NMR characterization, compared with the NMR spectrum of chlorinated polyether, the characteristic peak at 44.0-45.0ppm disappeared, indicating that the C-CI group was reacted; new characteristic peaks appeared at 25.5ppm and 35.2ppm, indicating that There are C-SH and C-Sx-C (X is 1-5) groups generated. The GPC characterization of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com