Pyrroloquinoline quinone derivative or medicinal salt thereof, preparation method and application
A technology of pyrroloquinoline quinone and its derivatives, which is applied in the field of biomedicine, can solve problems affecting drugability, etc., and achieve excellent solubility and ideal drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
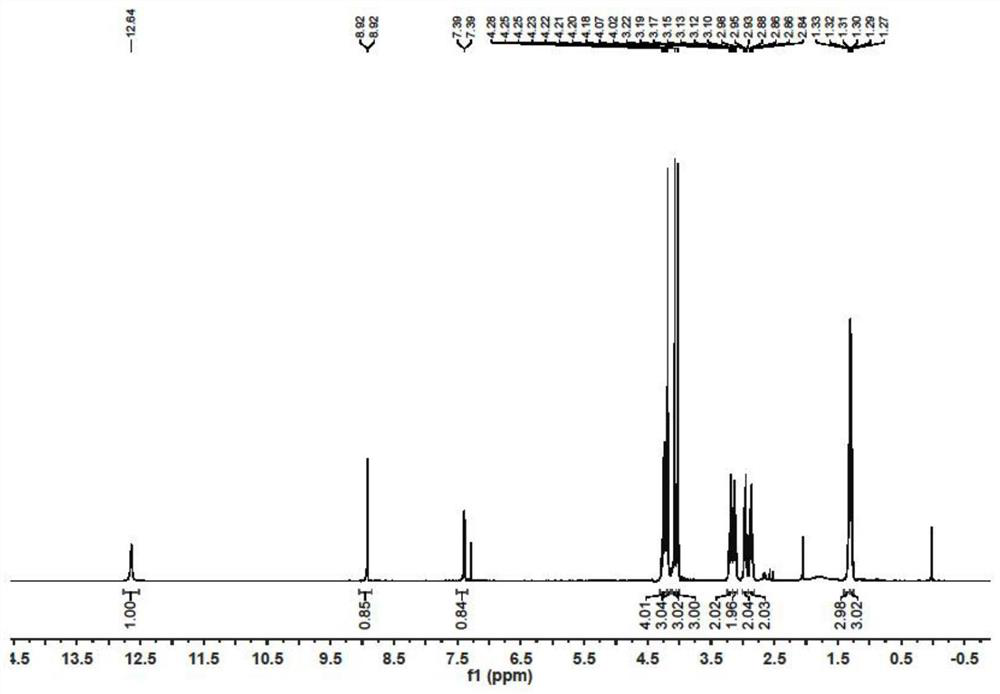
[0038] Synthesis of PQQ trimethyl ester: Take a single-neck flask, dissolve PQQ (3.3 g, 10 mmol) in DMF (200 mL), then add potassium carbonate (6.9 g, 50 mmol) in small portions and stir for 1 hour. Dimethyl sulfate (25 g) was dropped into the reaction suspension at room temperature, and the reaction was continued at room temperature for 5 days after the drop was completed. After DMF was evaporated with an oil pump, water (200 mL) was added to the residue and the residue was made acidic with 4N hydrochloric acid. The reaction solution was stirred at room temperature for 6 hours, and suction filtered to obtain an orange solid PQQ trimethyl ester (3.17 g, yield 85%).
[0039] Reduction of PQQ trimethyl ester: Take a single-neck flask, suspend PQQ trimethyl ester (1.86 g, 5 mmol) in acetonitrile (100 mL), add 1M sodium hydrosulfite solution (100 mL), and stir at room temperature overnight. The reaction solution was directly suction filtered, and the filter cake was dried to obtai...
Embodiment 2
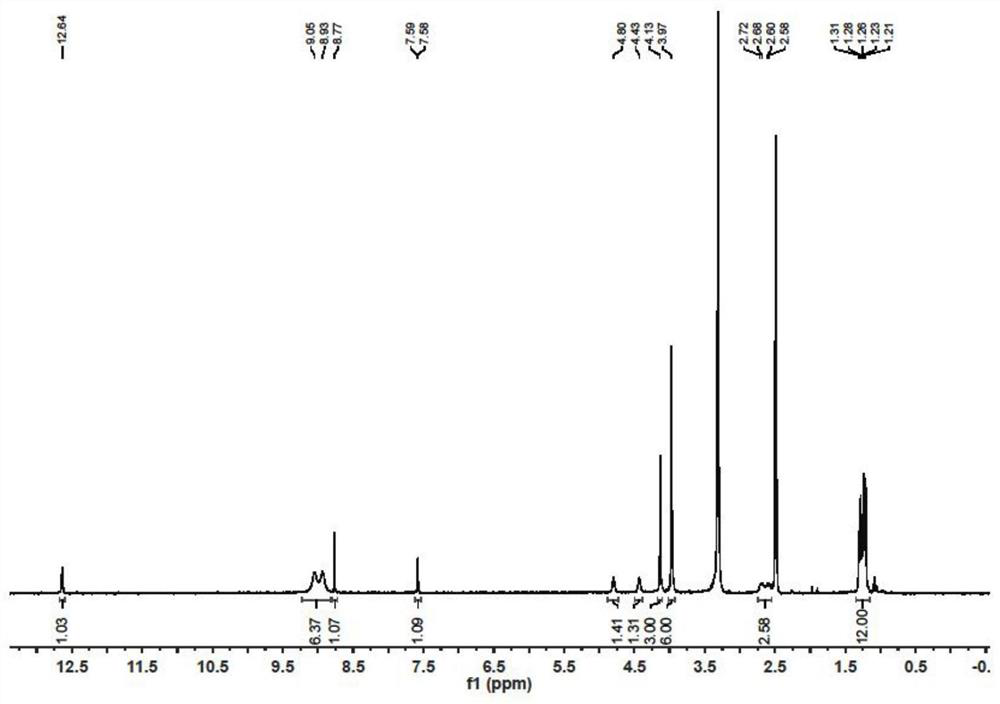
[0042] Referring to the method of Example 1, the acetyl chloride in Example 1 was replaced with palmitoyl chloride to obtain compound 2 (319 mg, 75%). 1 H NMR (300MHz, Chloroform-d) δ 12.67(s, 1H), 8.94(s, 1H), 7.32(s, 1H), 4.20(s, 3H), 4.06(s, 3H), 4.03(s, 3H), 2.83 (t, J=7.6Hz, 2H), 2.75 (t, J=7.5Hz, 2H), 1.91 (dt, J=14.3, 7.2Hz, 4H), 1.30 (d, J=8.3Hz, 54H), 0.90(t, J=6.3Hz, 6H).
Embodiment 3
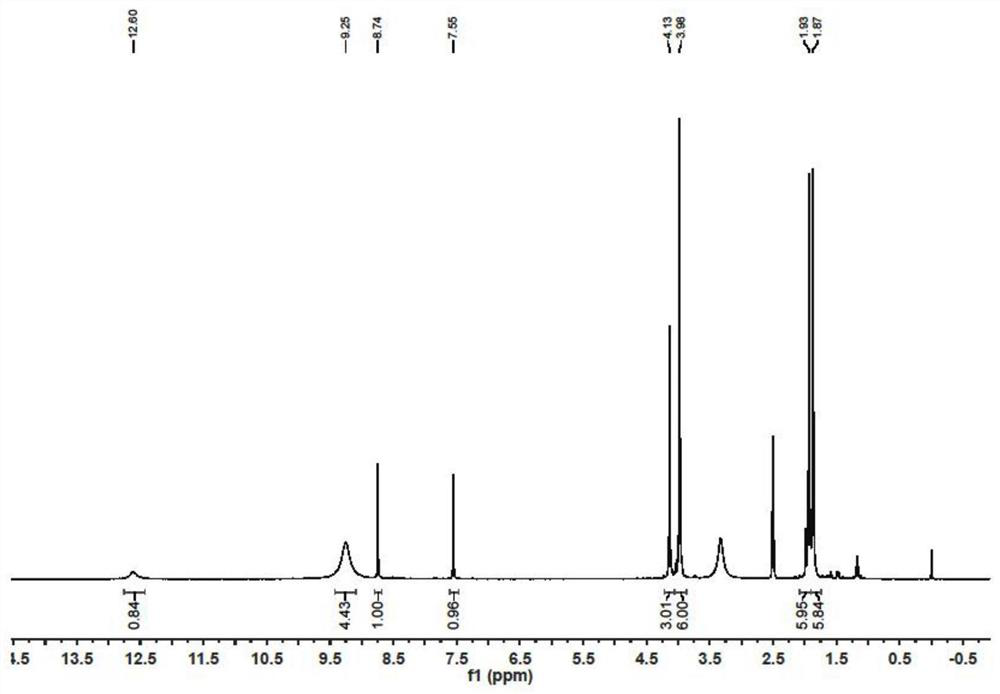
[0044]Referring to the method of Example 1, acetyl chloride in Example 1 was replaced with benzoyl chloride to obtain compound 3 (230 mg, 79%). 1 H NMR (300MHz, Chloroform-d) δ 12.74 (s, 1H), 8.96 (s, 1H), 8.47-8.11 (m, 4H), 7.95-6.91 (m, 7H), 4.21 (s, 3H), 4.01(s, 3H), 3.82(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com