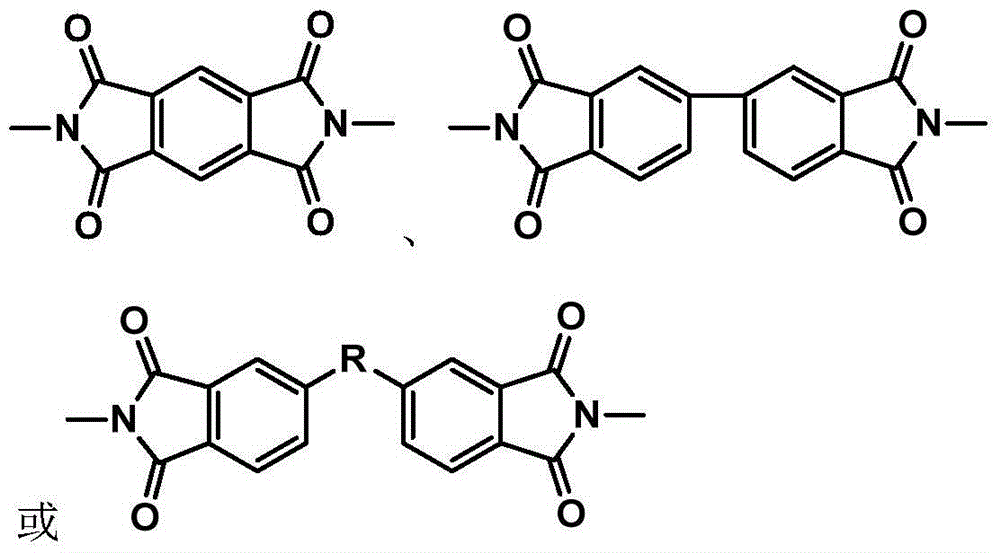
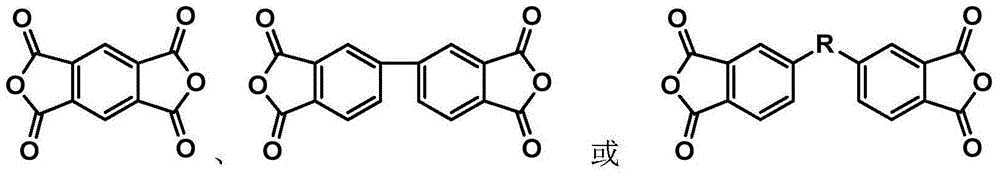
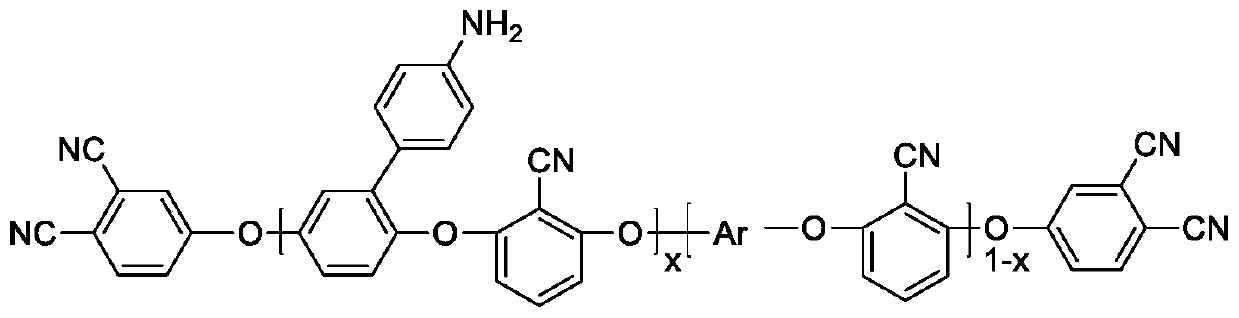
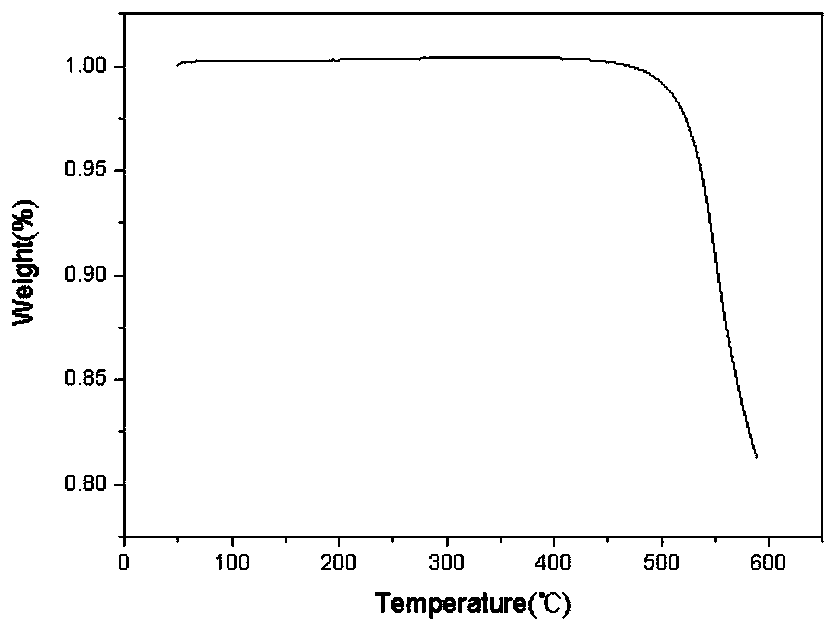
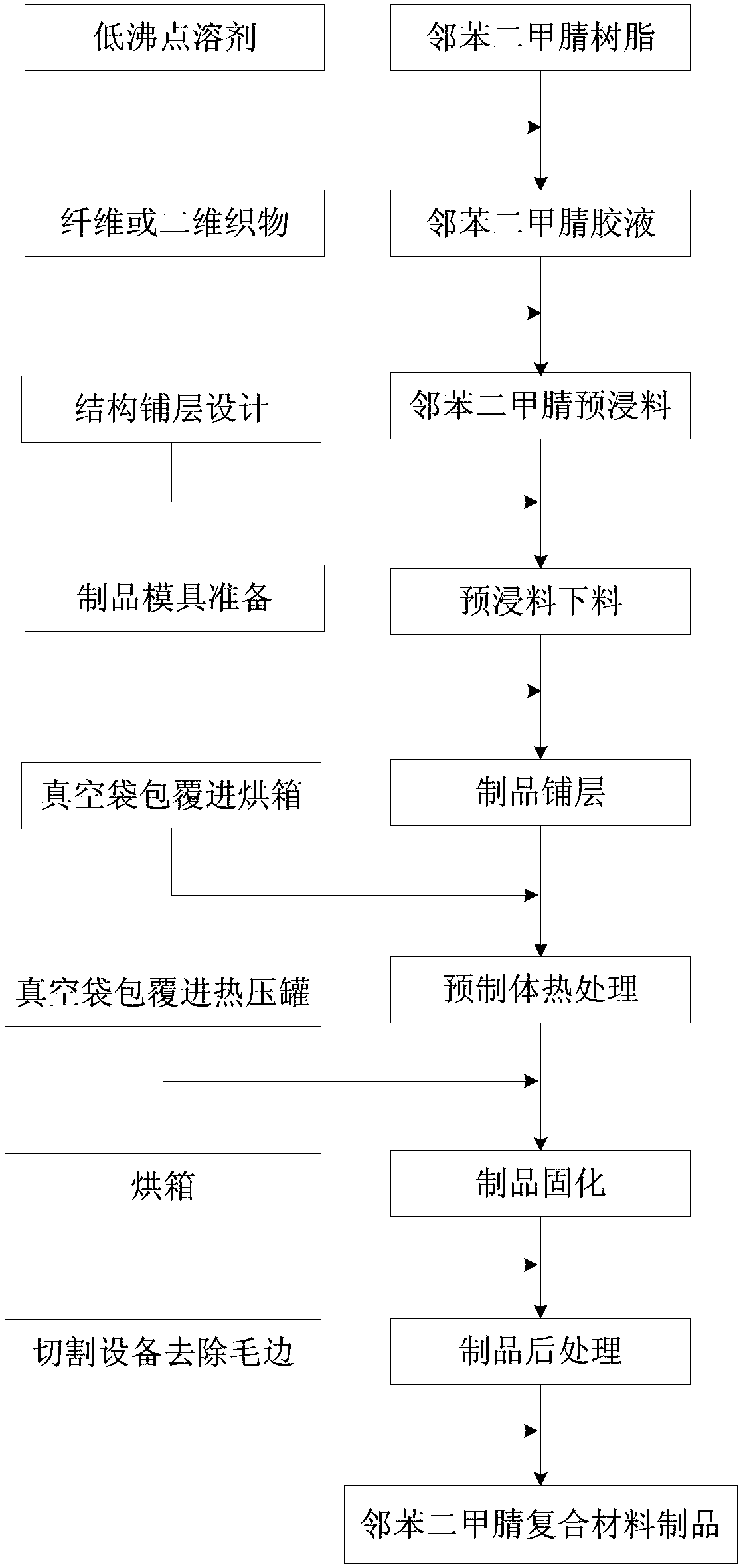
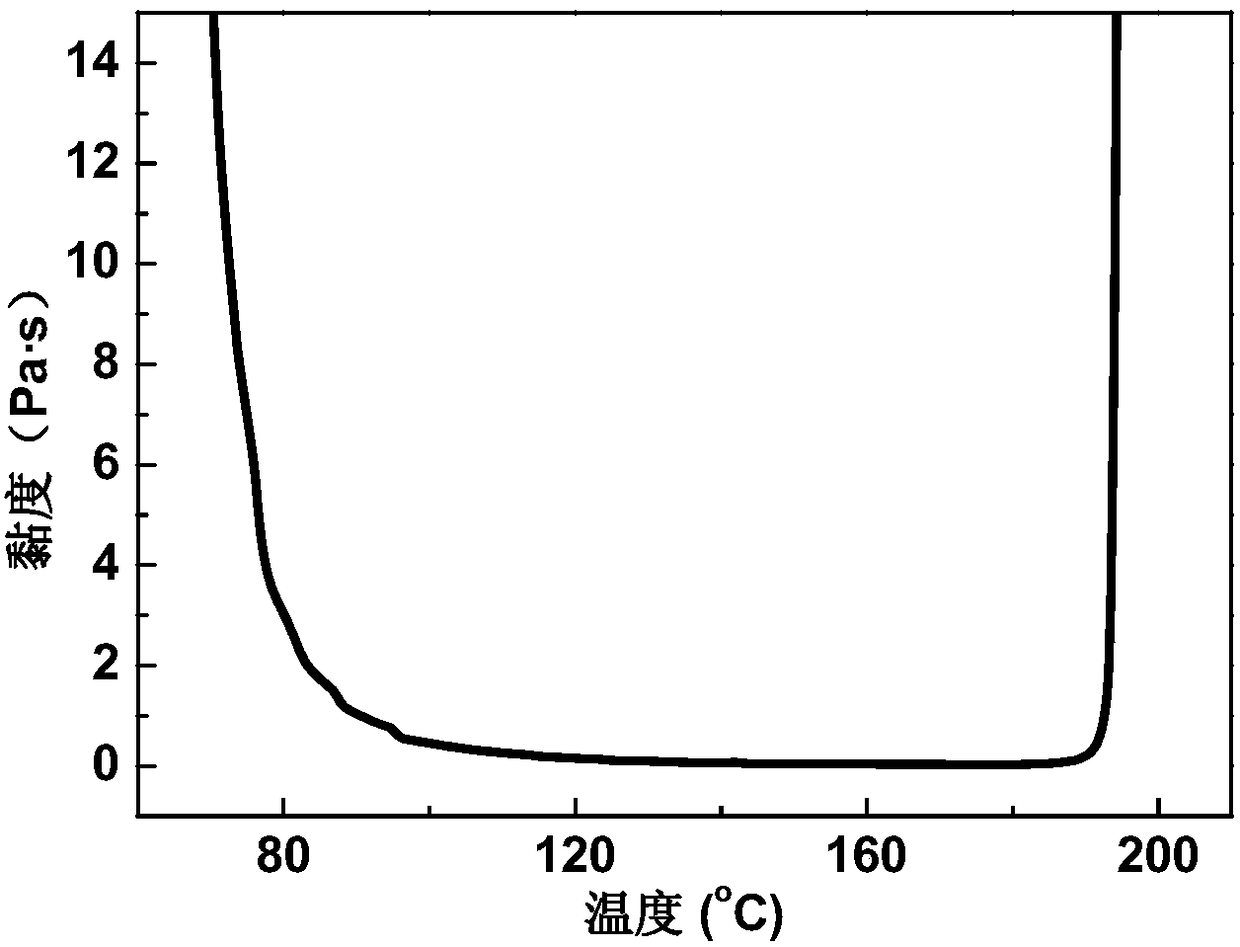
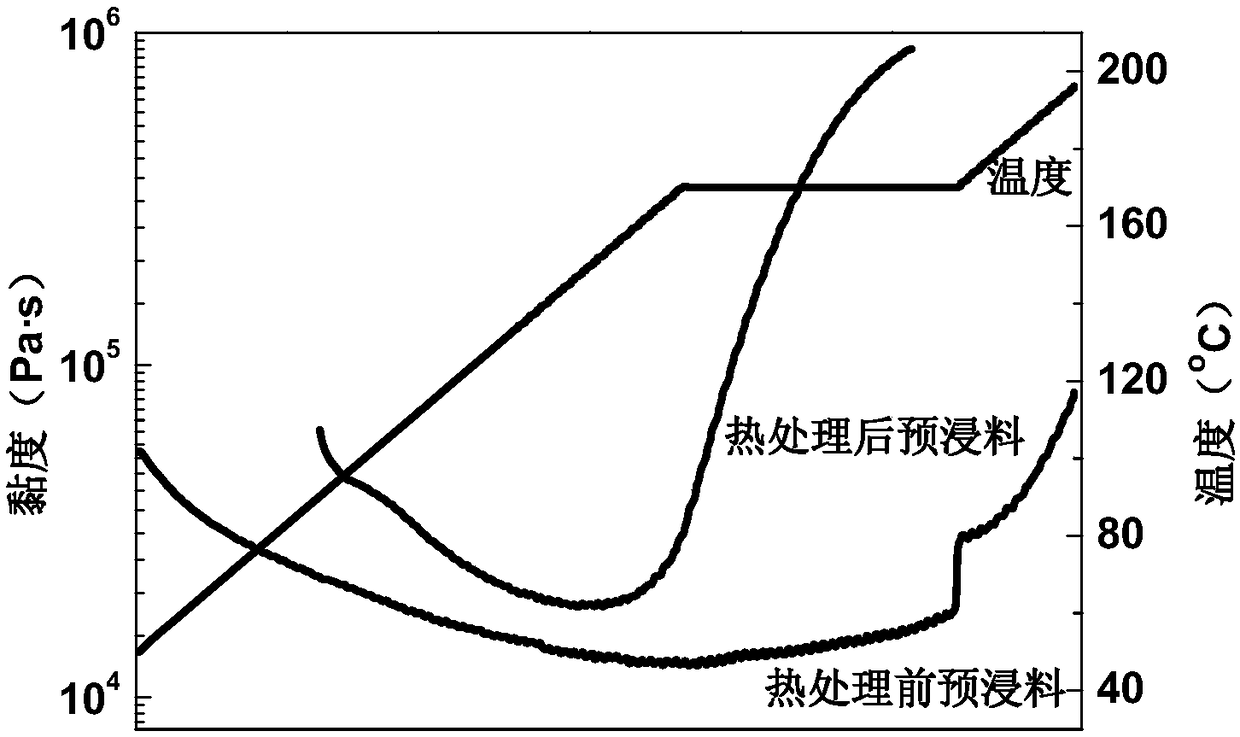
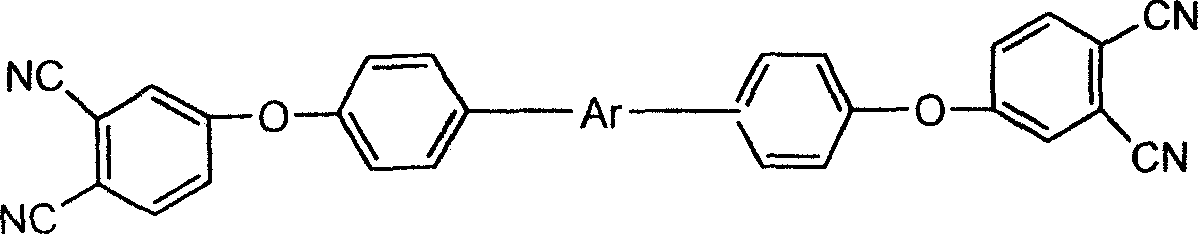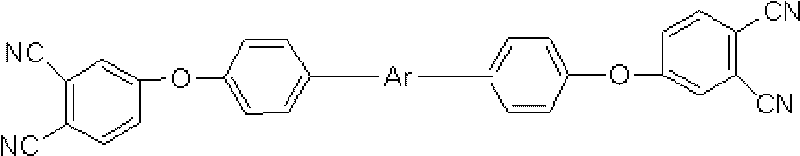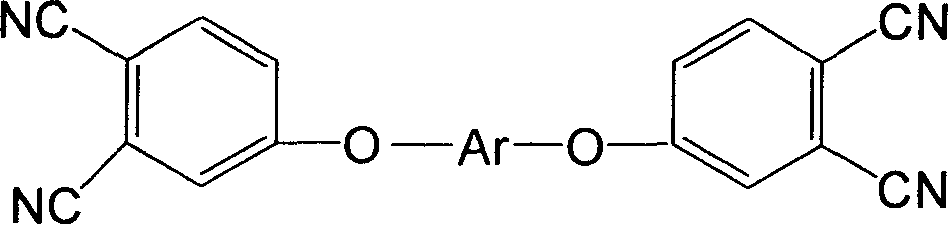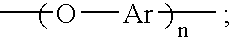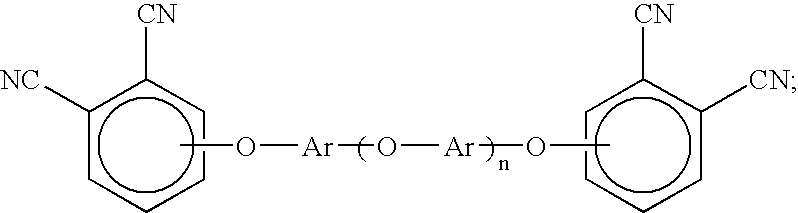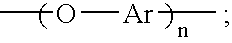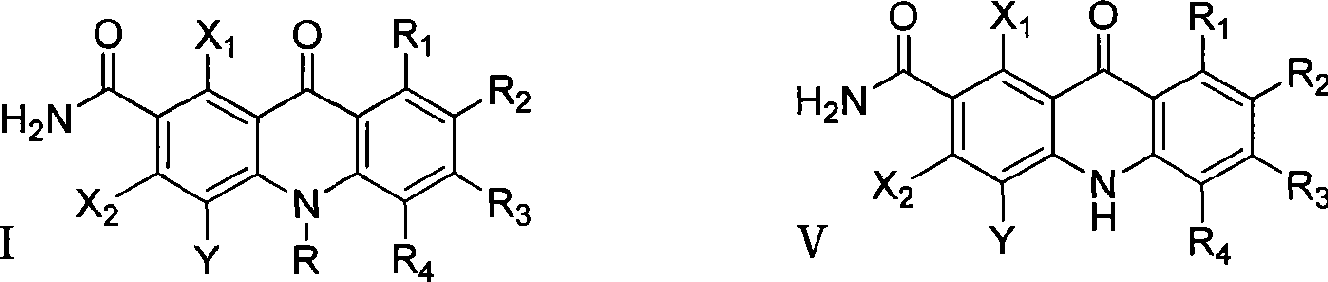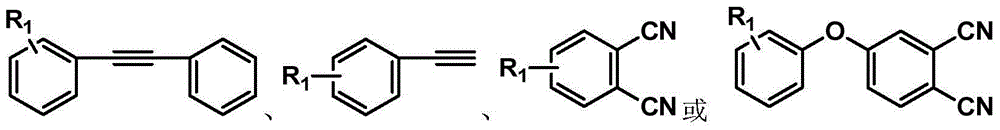Patents
Literature
427 results about "Phthalonitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phthalonitrile is an organic compound with the formula C₆H₄(CN)₂, which is an off-white crystal solid at room temperature. It is a derivative of benzene, containing two adjacent nitrile groups. The compound has low solubility in water but is soluble in common organic solvents. The compound is used as a precursor to phthalocyanine and other pigments, fluorescent brighteners, and photographic sensitizers.
Fluid diffusion layers
InactiveUS20070087120A1High char yieldMembranesSemi-permeable membranesHigh current densityPolymer science
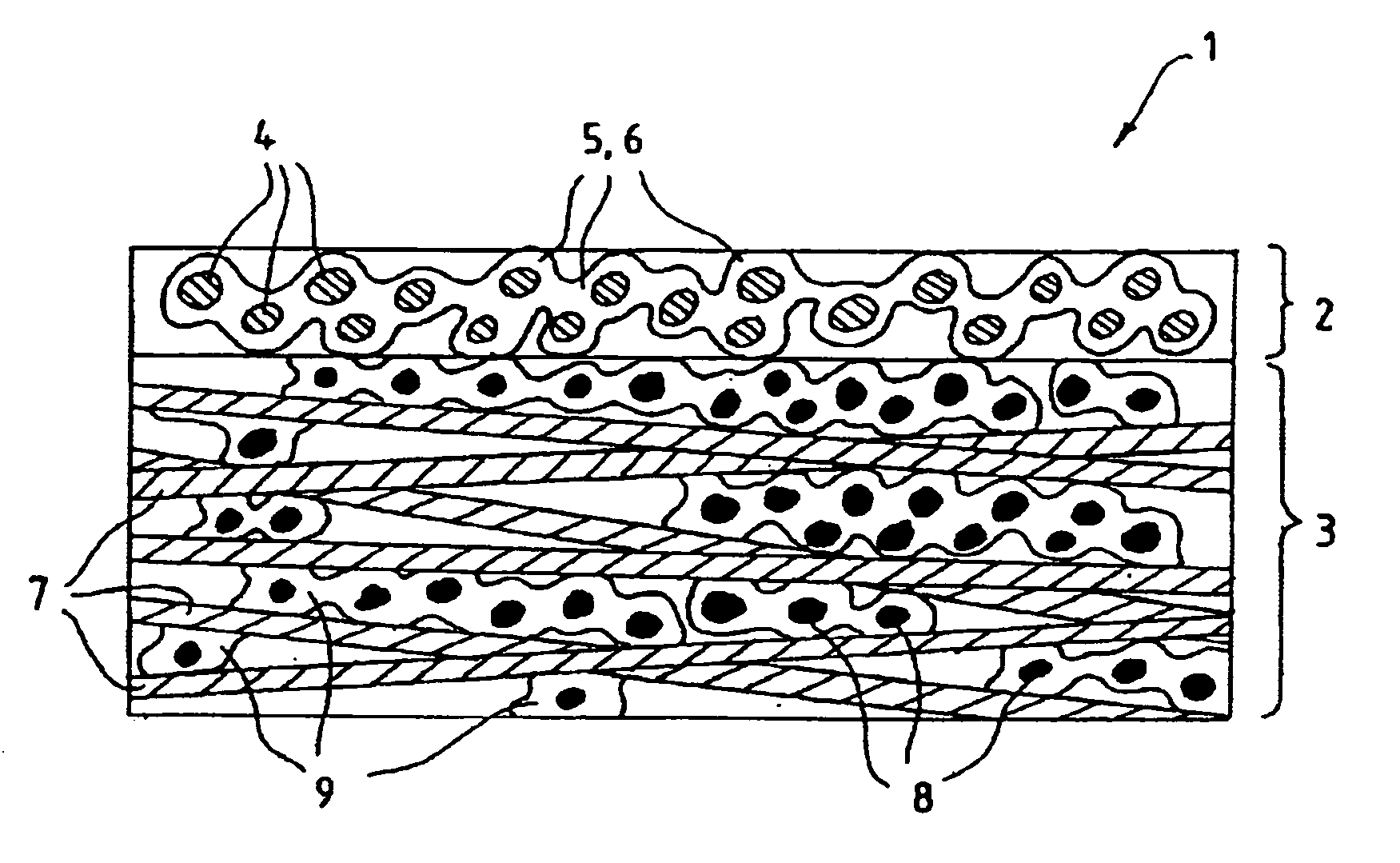
Fluid diffusion layers with favorable mechanical, physical and structural properties are prepared for fuel cell electrodes by: impregnating a porous carbonaceous web with a matrix comprising a polymer having pyrrolidone functionality and a high carbon char yield resin, such as activated aramid fiber pulp, lignins, phenolics, benzoxazines and phthalonitriles; and carbonizing the matrix. The polymer is optionally oxidized before carbonizing. The matrix may also include conductive fillers and / or pore formers. The fluid diffusion layers are particularly suitable for use in continuous roll-to-roll MEA processing of GDLs for use in solid polymer electrolyte fuel cells operating at high current densities and / or in highly humidified conditions.
Owner:BDF IP HLDG
Double end group ortho-benzene dimethyl nitrile-benzo oxazine resin, solidified substance and its preparing method and use
InactiveCN1944400ARegulating and Controlling Control StructuresRegulating and Controlling Control PerformanceCarboxylic acid nitrile preparationOrganic compound preparationBenzeneHeat resistance
The present invention belongs to the field of high molecular synthetic material, and is especially one kind of double end group o-phthalonitrile-benzo oxazine resin, and its solidified substance and preparation process. The present invention provides one kind of 4-aminophenoxyl-o-phthalonitrile monomer and the double end group o-phthalonitrile-benzo oxazine resin therewith. The double end group o-phthalonitrile-benzo oxazine resin has excellent curing reaction activity, low temperature curing performance similar to that of benzo oxazine resin, heat resistance similar to that of o-phthalonitrile resin, greatly lowered curing temperature and capacity of use in high temperature condition. The preparation process is simple and controllable, and the product has excellent application foreground.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
Covalence triazine organic polymer visible-light-driven photocatalyst and preparing method and application thereof
ActiveCN104525258AImprove stabilityMild preparation conditionsWater/sewage treatment by irradiationOrganic-compounds/hydrides/coordination-complexes catalystsAcid catalysisTriflic acid
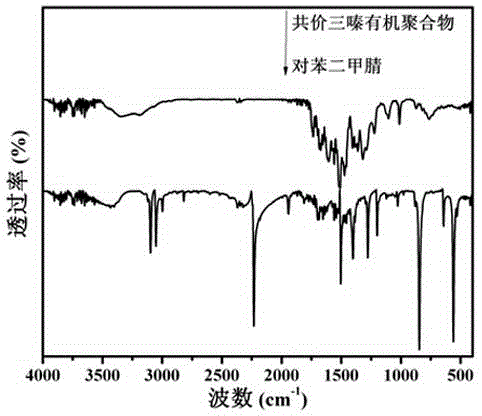
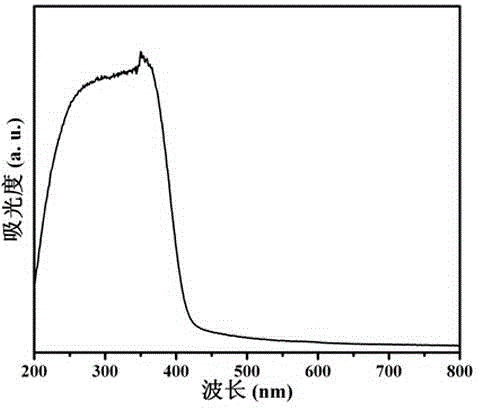
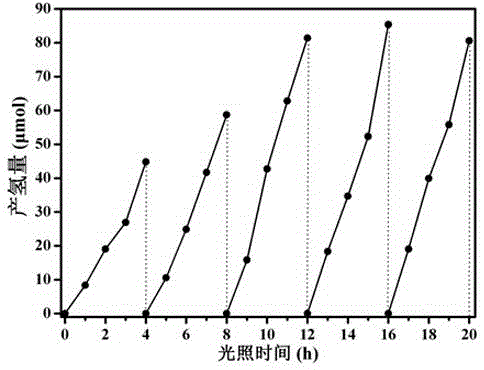
The invention discloses a covalence triazine organic polymer visible-light-driven photocatalyst and the preparing method and application thereof and belongs to the technical field of material preparation and photocatalysis. According to the method, normal-temperature liquid-phase polymerization is adopted, para-phthalonitrile serves as the monomer, trimerization is conducted under the catalysis of trifluoromethanesulfonic acid, and then the covalence triazine organic polymer visible-light-driven photocatalyst is prepared. By means of the photocatalyst, visible-light reaction is achieved, hydrogen production through water photolysis can be achieved without obvious inactivation, and organic pollutants in waste water can be effectively degraded. The preparing condition is mild, production cost is low, yield is high, actual production requirements are met, and application potential is great.
Owner:FUZHOU UNIV
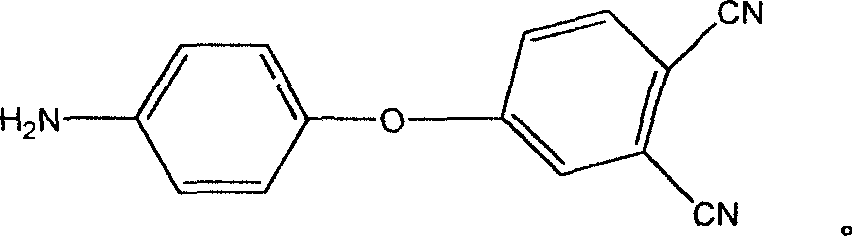
Aromatic diamine with phthalonitrile pendant group, preparation method thereof and polyimides or polyamide prepared therefrom
InactiveCN101307013ARegulating processabilityRegulatory usabilityPreparation by carboxylic acid amide dehydrationFiberSide chain
The invention discloses an aromatic diamine containing o-phthalonitrile side group. The structure formula of the aromatic diamine is shown as the right formula. The invention also discloses a method for preparing the aromatic diamine and polyimide and daiamid which are prepared by taking the aromatic diamine containing the o-phthalonitrile side group as one of raw materials. The o-phthalonitrile side group in the aromatic diamine is positioned on the side chain, thereby adjusting the degree of crosslinking with adjusting the polymer molecular weight and widening the application of the polyimide and the daiamid in a thick-wall composite material or a composite material element with a complicated shape and the fields of membrane material and fiber.
Owner:SICHUAN UNIV
Aromatic diamine containing phthalonitrile side group and synthesis method and application thereof
InactiveCN102993070AIncrease typeBreak the rigid structureCarboxylic acid nitrile preparationOrganic compound preparationImidePolymer science
The invention discloses aromatic diamine containing phthalonitrile side group, which is characterized in that the structural formula is shown in the specification, wherein R is one of CF3CCF3, CH3CCH3, O, CO and S. A preparation method of the aromatic diamine containing phthalonitrile side group comprises the following steps of: adding 4-nitrophthalonitrile or 3-nitrophthalonitrile, a compound 1, potassium carbonate and a high-boiling point solvent into a reaction container; conducting reaction for 10-24 hours at room temperature under protection of nitrogen; and then precipitating, filtering and drying. The aromatic diamine containing phthalonitrile side group can be applied to the preparation of polyimide, polyamide and polyamide-imide.
Owner:SICHUAN UNIV
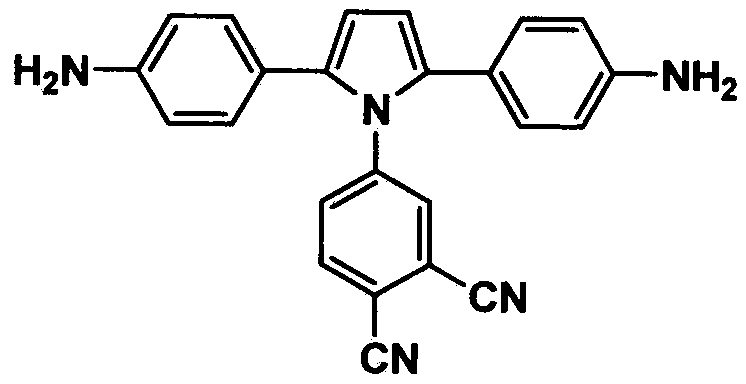
Pyrrole aromatic diamine containing phthalic nitrile structure as well as preparation method and application thereof
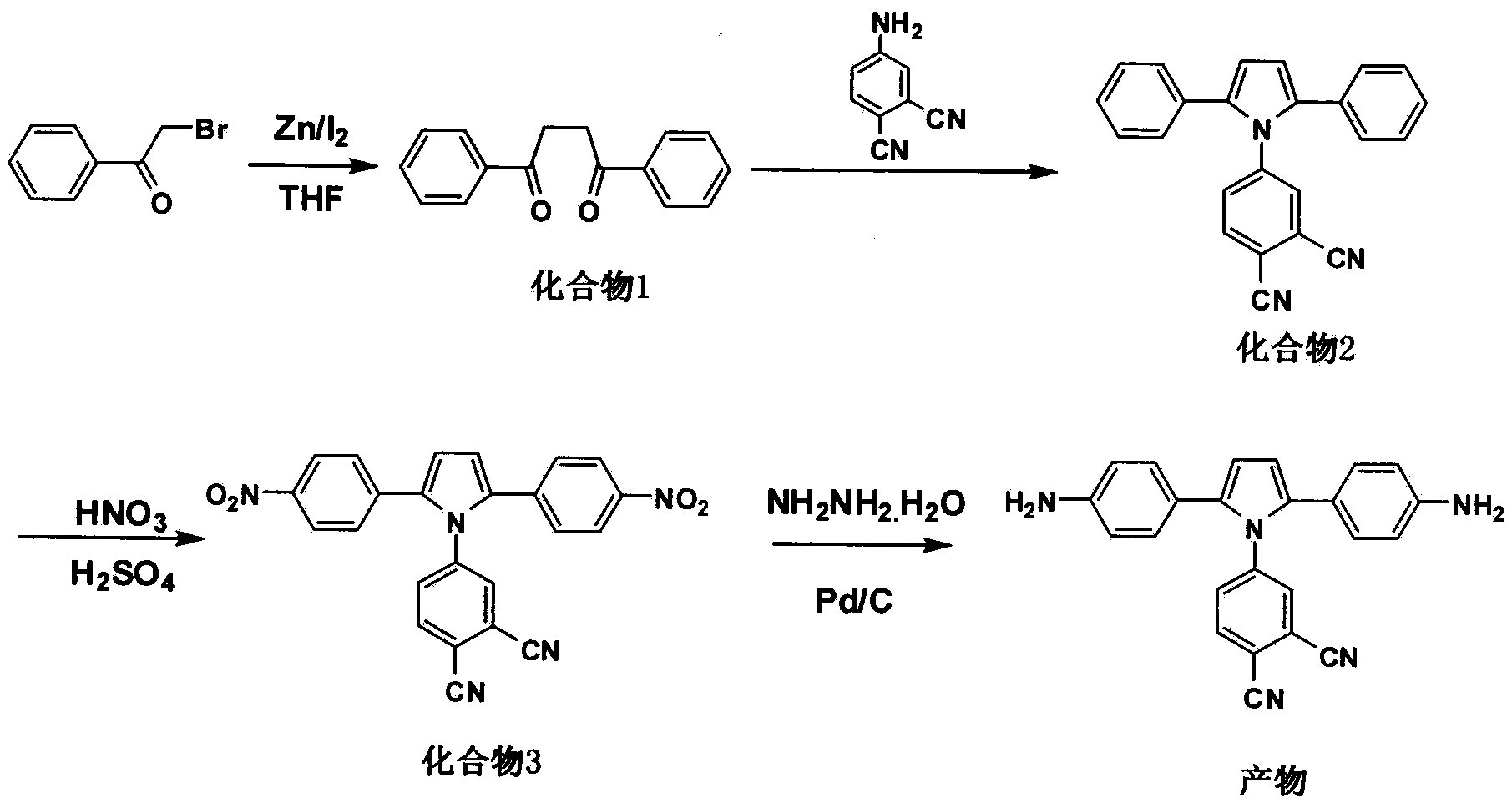
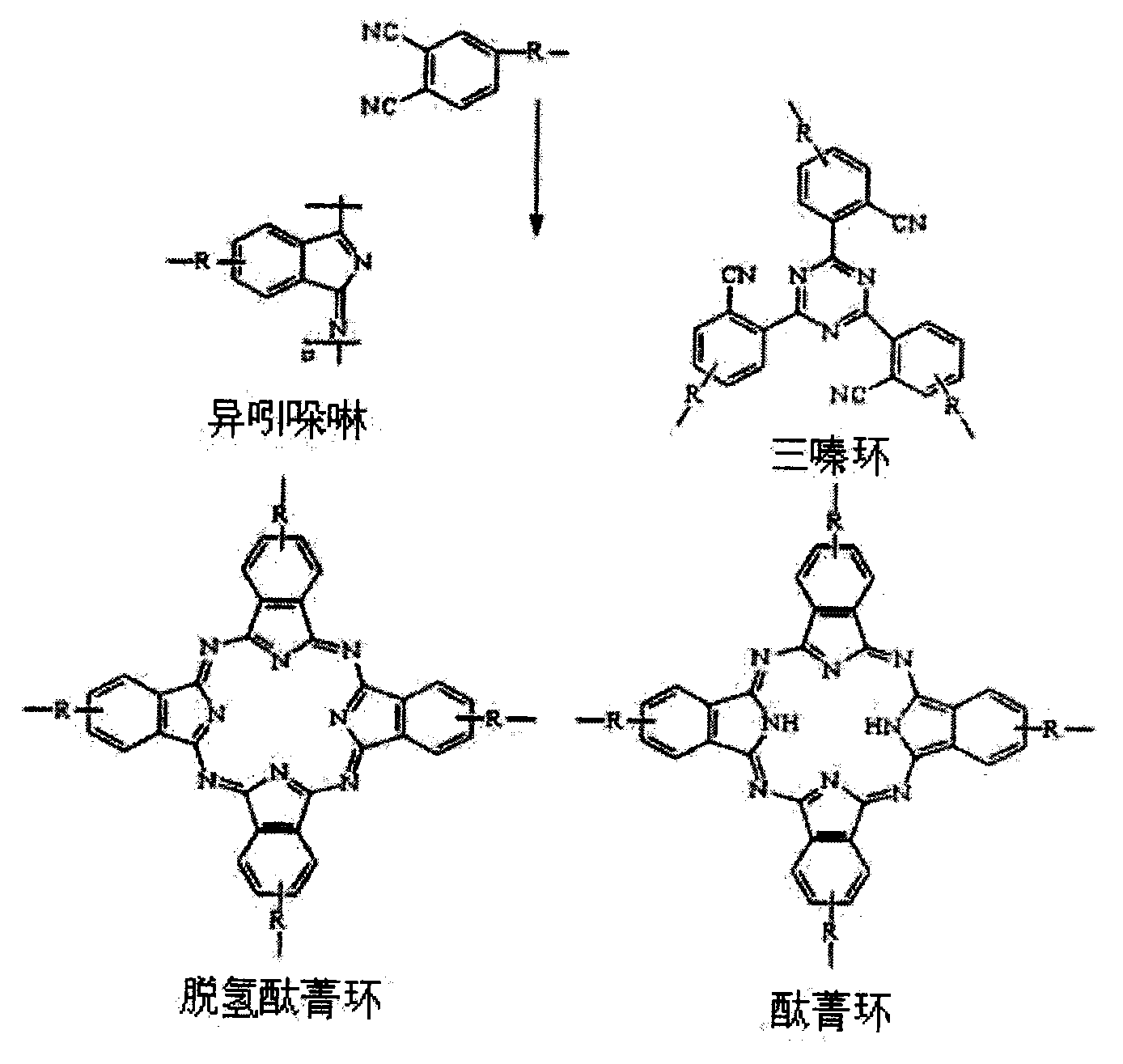
The invention discloses a pyrrole aromatic diamine containing a phthalic nitrile structure and a preparation method thereof. 2-bromoacetophenone is taken as a raw material to prepare 4-(2,5-bi(4-aminophenyl)-pyrryl) phthalonitrile by reaction of four steps, namely condensation, closed loop, nitration and reduction. The aromatic diamine disclosed by the invention is high in purity, and stable at room temperature, and is an important monomer component for preparing a plurality of polyimides, and an application of the polyimides in the fields such as a high-performance fiber, organic luminescent materials, a functional coating, a special adhesive and the like is expanded.
Owner:四川启科新材料有限责任公司
Method for preparing three-dimensional nitrogen-doped graphene/CoOx composite material
InactiveCN104319395AEasy to prepareEvenly dispersedMaterial nanotechnologyCell electrodesDoped grapheneActive agent
The invention relates to a method for preparing a three-dimensional nitrogen-doped graphene / CoOx composite material, and belongs to the technical field of functional materials. The method comprises the main processes of: by taking phthalonitrile as a nitrogen-containing precursor, cobalt acetate as a transition metal precursor, and graphene oxide prepared by the Hummers method as a carbon carrier, adding a surface active agent and carrying out solvent-thermal-technology one-step in-situ coordination, assembling, freeze drying and calcination treatment to obtain the three-dimensional structured nitrogen-doped graphene / CoOx composite nano material. In comparison with the prior art, the three-dimensional nitrogen-doped graphene / CoOx composite material prepared by the invention has the advantages that particles are homogeneous and do not agglomerate; in addition, more electron transmission channels exit because of mesopores left after the surface active agent is removed, the impedance is reduced, and the electrochemical property of the material is improved.
Owner:SHANGHAI UNIV
Poly-o-phthalonitrile resin and its preparing process
The precent invention discloses a poly-o-phthalonitrile resin and a preparetion method. After the solution blending, fusion blending or physical blending of 1 to 99 percent of o-phthalonitrile derivative with amino group or hydroxyl group and 1 to 99 percent of polyethernitrile with amino group or hydroxyl group, or 1 to 99percent of o-phthalonitrile derivative with amino group or hydroxyl group and 1 to 99 percent of o-phthalonitrile derivative, or 1 to 99 percent of polyethernitrile with amino group or hydroxyl group and 1 to 99 percent of o-phthalonitrile derivative, or 1 to 98 percent of o-phthalonitrile derivative with amino group or hydroxyl group, 1 to 98 percent of polyethernitrile with amino group or hydroxyl group and one to ninety eight percent of o-phthalonitrile derivative, the compound is solidified, so that the poly-o-phthalonitrile resin is prepared. Because the invention uses the o-phthalonitrile derivative with amino group or hydroxyl group or / and the polyethernitrile to prepare the poly-o-phthalonitrile resin, the performance of the resin can be regulated and controlled in a wide range, and the tenacity of the poly-o-phthalonitrile resin can also be increased, thus broadening the application field of the resin. The invention also has the advantages of simple steps and low cost.
Owner:SICHUAN UNIV
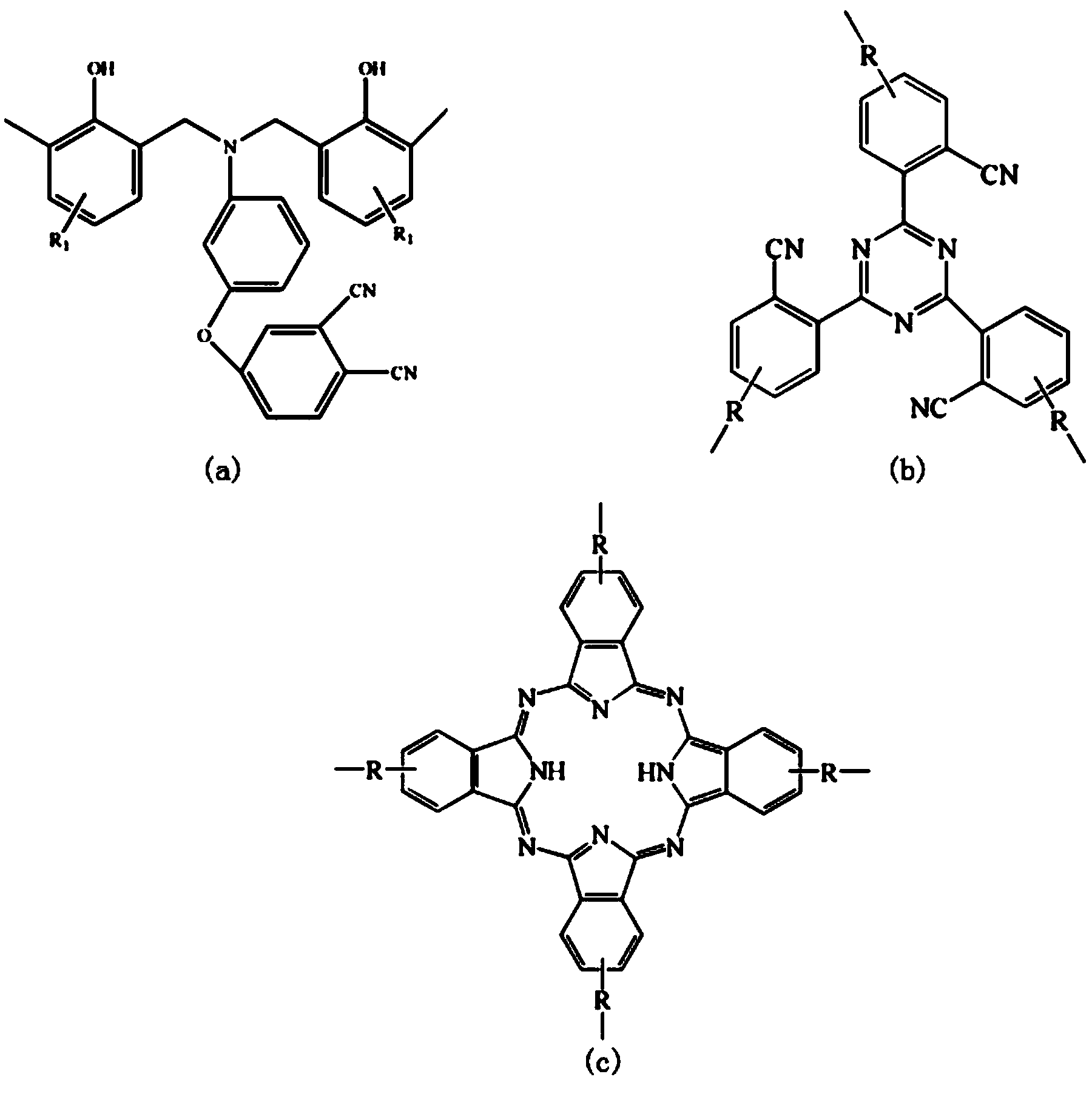
Thermosetting resin glass fiber laminating composite material and preparation method thereof
ActiveCN101700705AImprove thermal stabilityHigh mechanical strengthShieldingGlass/slag layered productsFiberGlass fiber
The invention belongs to the field of high molecular material and in particular to a thermosetting resin glass fiber laminating composite material and a preparation method thereof; the solved technical problem is that the thermosetting resin glass fiber laminating composite material which can adsorb electromagnetic waves is provided, and the thermosetting resin glass fiber laminating composite material is prepared by compounding and laminating double-terminal-group phthalonitrile-benzoxazine resin, which is shown by formula I, filling material, low boiling polar solvent and glass fiber cloth;the filling material is micron-sized carbonyl iron dust or multi-walled carbon nanotube. The thermosetting resin glass fiber laminating composite material has the characteristic that the mechanical structure performance and the electromagnetic wave adsorption performance are integrated, the material is applied to the electromagnetic wave adsorption material, compared with the carbon fiber, the glass fiber is used as reinforcing material, the cost is low, and the processing production is easy.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Bi-phthalonitrile prepolymer as well as preparation method and application thereof, bi-phthalonitrile prepolymer/epoxy resin copolymer and preparation method thereof as well as bi-phthalonitrile prepolymer/epoxy resin cured material
ActiveCN102492140AOvercoming the defects limited by high temperature useImprove flame retardant performanceOrganic chemistryEpoxyPolymer science
The invention belongs to the technical field of a high polymer material, and in particular relates to a bi-phthalonitrile prepolymer as well as a preparation method and application thereof, a bi-phthalonitrile prepolymer / epoxy resin copolymer and a preparation method thereof as well as a bi-phthalonitrile prepolymer / epoxy resin cured material. According to the invention, the structural formula ofthe bi-phthalonitrile prepolymer is shown in a formula I described in the specification; and the bi-phthalonitrile prepolymer can be used as a high polymer material flame retardant and is copolymerized with epoxy resin so as to prepare the bi-phthalonitrile prepolymer / epoxy resin copolymer and the bi-phthalonitrile prepolymer / epoxy resin cured material, wherein the flame retardant property of theobtained bi-phthalonitrile prepolymer / epoxy resin cured material is greatly improved, and simultaneously, the good heat resistance and physical properties such as high strength and high modulus of the obtained bi-phthalonitrile prepolymer / epoxy resin cured material are also ensured, namely, the comprehensive properties of the obtained bi-phthalonitrile prepolymer / epoxy resin cured material are excellent.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
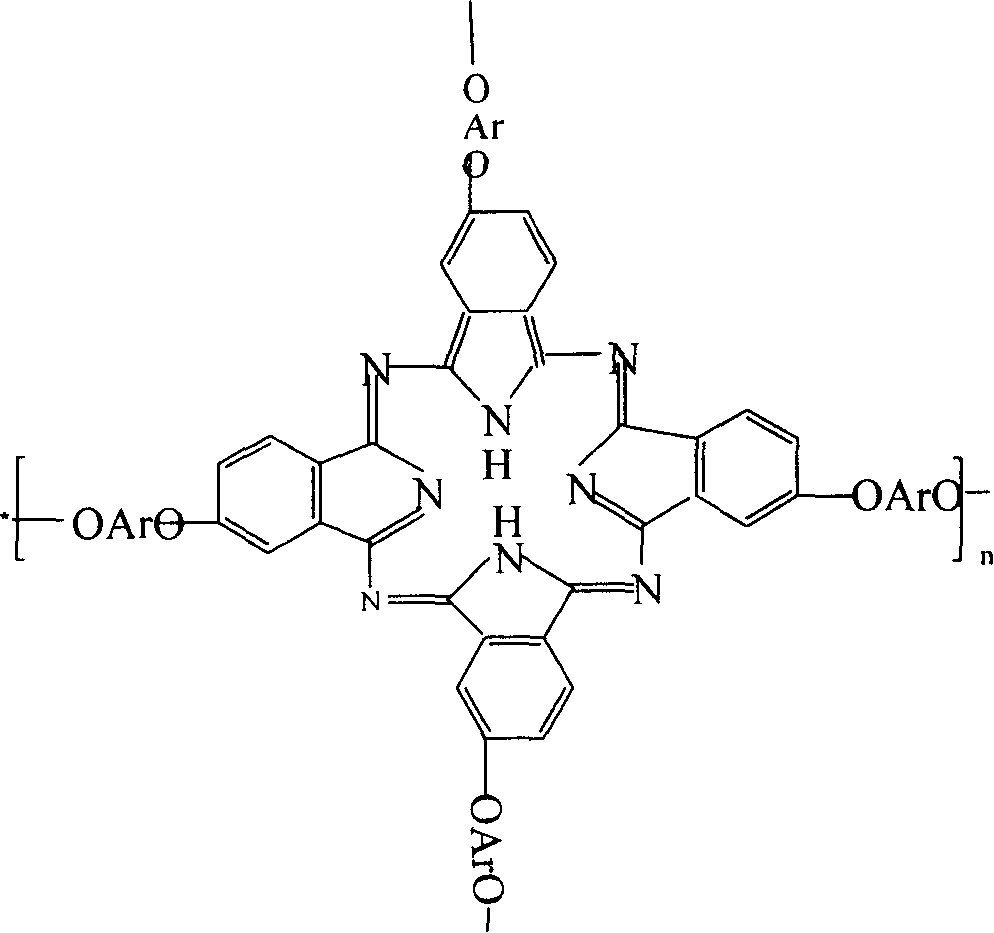
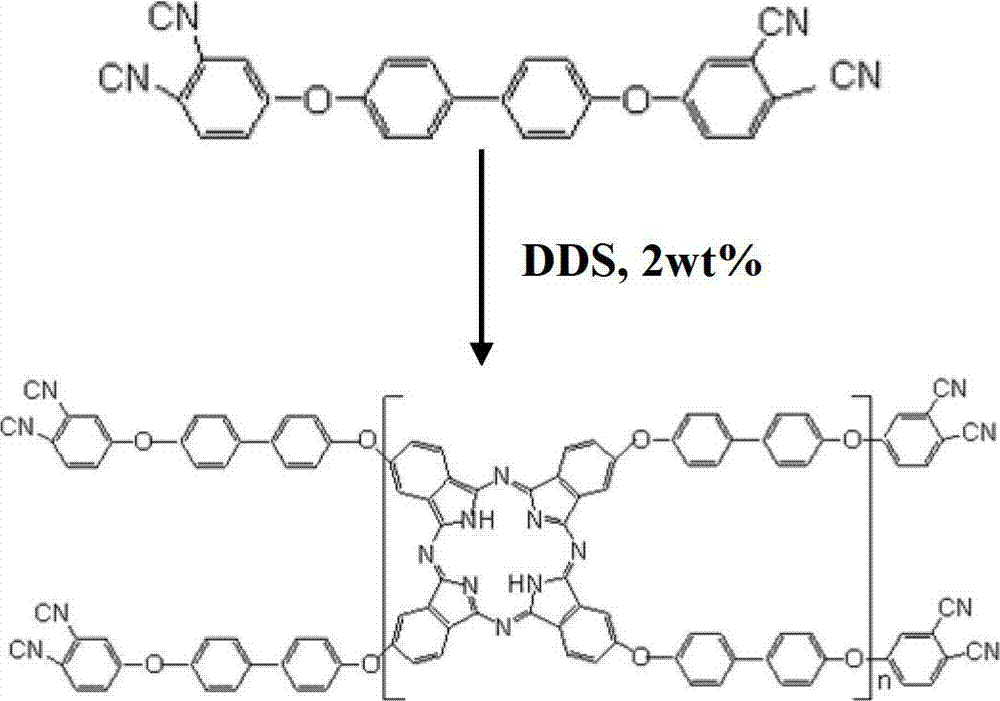
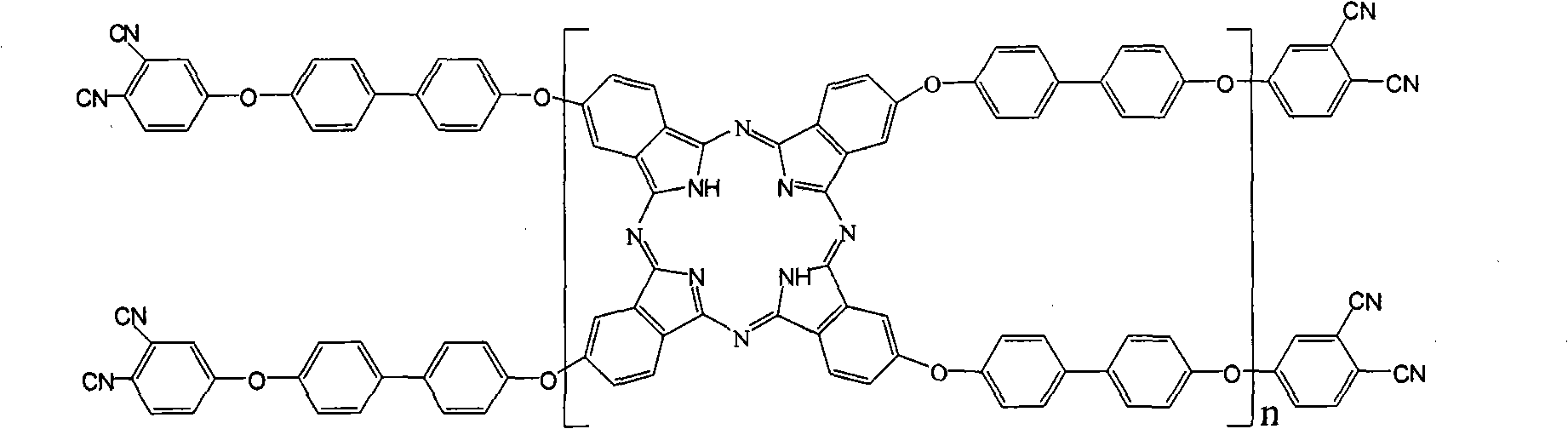
Double end-group phthalonitrile, resin, condensate and its preparation method and uses
ActiveCN1876615ARegulatory and Control StructuresTuning and Controlling PerformanceOrganic chemistryPolymer scienceEnd-group
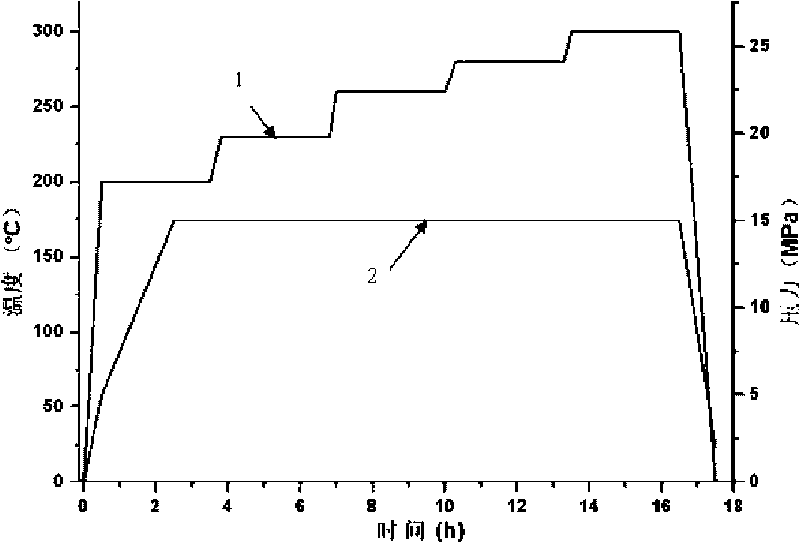
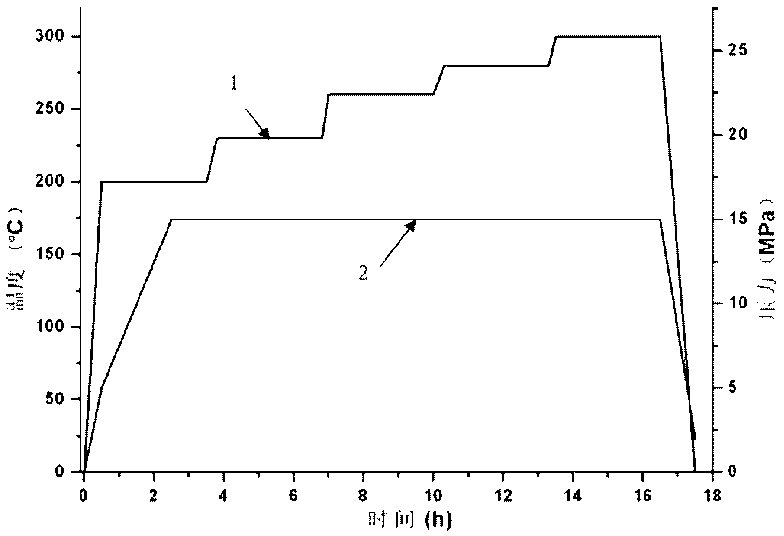
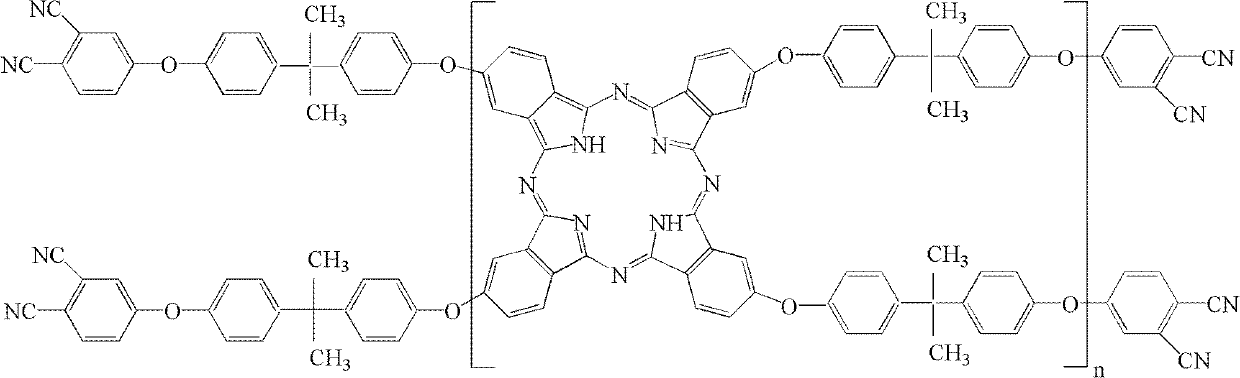
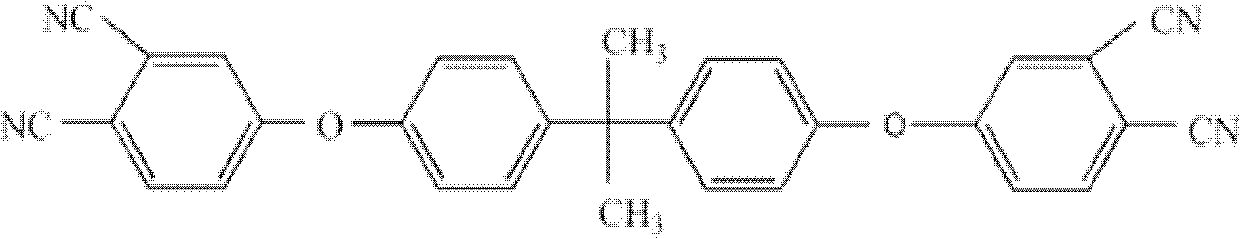
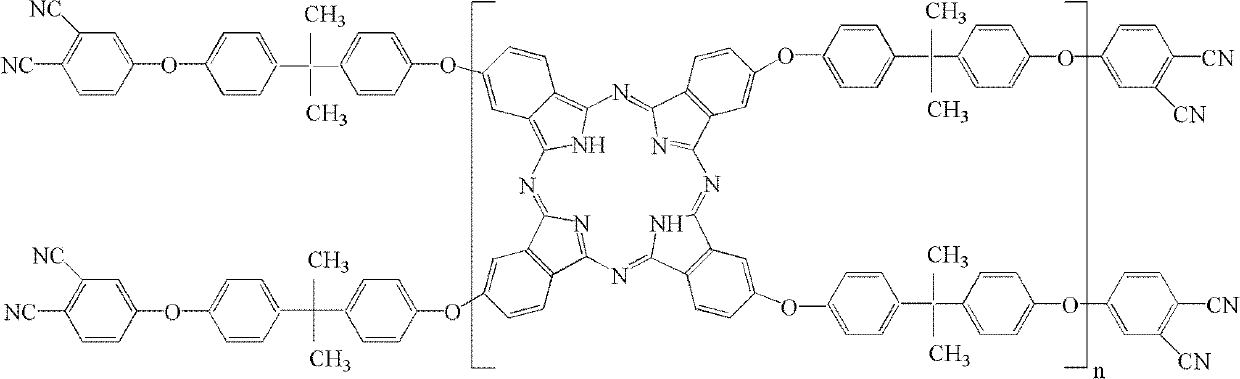
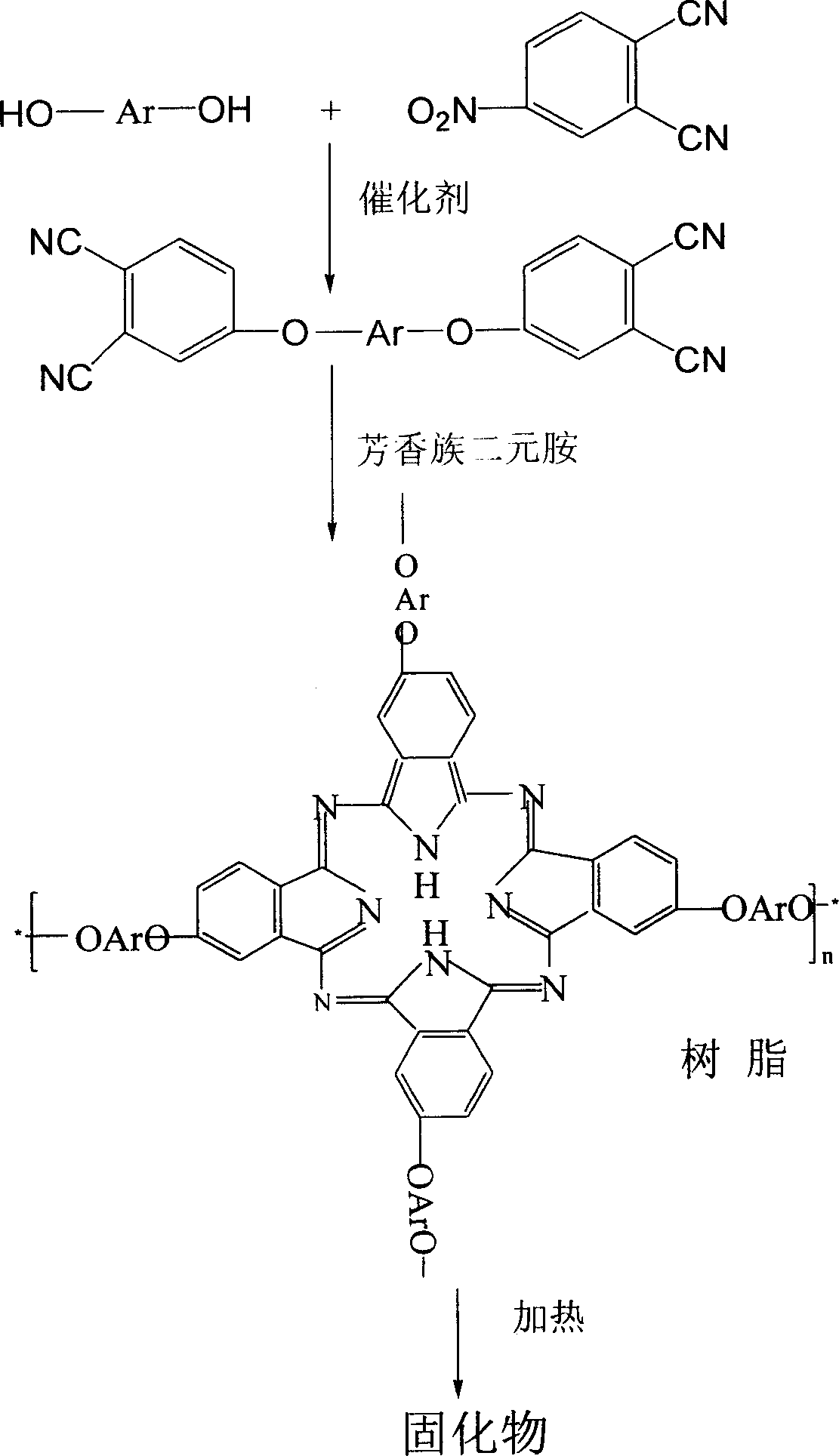
The invention belongs to the high molecular synthetic material field. The invention relates the double-end phthalonitrile, resin, condensate, which are made by phthalonitrile, and preparing method. The invention uses 4-nitro-o-phthalonitrile and dihydric phenol as reaction integral to get double-end phthalonitrile. The double-end phthalonitrile and diamine are used to react in aromatic amine solution at 140-200Deg.C to get phthalonitrile resin which possesses phthalocyanine ring structure. The resin is cured at 220-280Deg.C, then carried out heat treatment at 320-370Deg.C to get phthalocyanine condensate which possesses high heat stability. The invention has the advantages of good heat stability and fire-retardancy.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
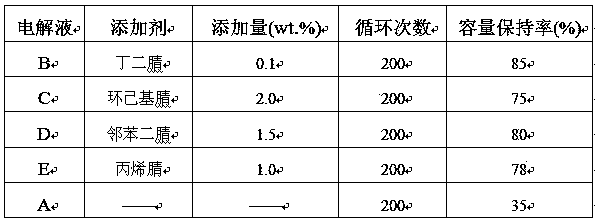
Electrolyte for 4.5 V lithium ion battery
InactiveCN103441303AImprove high-pressure cycle performanceImprove cycle performanceSecondary cellsElectrolytic agentAcrylonitrile
The invention relates to an electrolyte for a 4.5 V lithium ion battery. The electrolyte comprises the following components in percentage by weight: 80%-89% of organic solvents, 10%-15% of lithium salt and 0.1%-5.0% of additives, wherein the additives are nitrile compounds, and the nitrile compounds include one of butanedinitrile, adiponitrile, sebaconitrile, acrylonitrile, cyclohexyl nitrile, 1,2-cyclohexyl dinitrile, phthalonitrile and cyanopyridine. According to the invention, the additives effectively improve the high-pressure cycle property of the lithium ion battery, can enhance the voltage of a common electrolyte to 4.5 V and are obvious in effect. The electrolyte disclosed by the invention is difficult to decompose under high voltage of 4.5 V, obviously enhances the cycle property of the lithium ion battery, and less influences the battery capacity.
Owner:嘉德力电源科技(苏州)有限公司
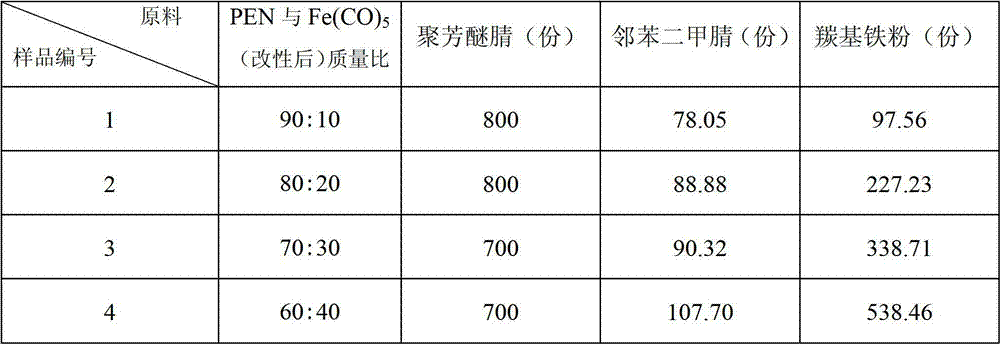
Polyaryl ether nitrile (PEN) and carbonyl iron powder (Fe(CO)5) composite magnetic material and preparation method thereof
ActiveCN102775755AImprove mechanical propertiesHigh strengthOrganic/organic-metallic materials magnetismPigment treatment with macromolecular organic compoundsPhthalonitrileMechanical property
The invention discloses a PEN and Fe(CO)5 composite magnetic material and a preparation method thereof, and belongs to the technical field of magnetic polymer materials. PEN serves as an organic matrix, Fe(CO)5 serves as an inorganic filler, and the composite magnetic material is obtained after blending and prilling. Firstly, the Fe(CO)5 is subjected to surface modification, and a layer of phthalonitrile prepolymer is generated on the surface of the Fe(CO)5; due to a layer of organic matters rich in cyan coated on the surface, the Fe(CO)5 after surface modification can improve the interfacial adhesive force between the Fe(CO)5 inorganic filler and the PEN organic matrix, so that the PEN and the Fe(CO)5 are provided with a high-strength magnetic property while maintain an excellent mechanical property of the PEN after composition. According to the magnetic material and the preparation method thereof, the Fe(CO)5 can be filled as much as possible on the premise that the good mechanical property of the composite material is maintained, so that magnetic saturation strength of the composite material can be improved, the compatibility of inorganic fillers with polymers is improved, and processing defects are overcome.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Porous phthalonitrile resin and preparation method thereof, and application of resin
ActiveCN105566629AThe preparation process is environmentally friendlyEfficient preparationDecompositionResin matrix
The invention discloses a porous phthalonitrile resin and a preparation method thereof, and application of the resin. The resin is prepared by blending the following composition and carrying out reaction and curing. The composition comprises the following components in parts by weight: 20-100 parts of phthalonitrile, 1-80 parts of pore-forming agent, 1-20 parts of catalyst and 2-20 parts of optional thermoplastic high molecule. The phthalonitrile resin forms pores due to the decomposition of the pore-forming agent in the curing process, and thus, the invention provides a simple environment-friendly high-efficiency method for preparing the porous material. The appropriate phthalonitrile matrix and pore-forming agent can be selected to implement the control on the pore structure. The porous phthalonitrile resin disclosed by the invention has excellent heat stability and mechanical properties, can be used as a high-performance composite material resin matrix, and has application values in the field of aerospace.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Low-viscosity cyano resin monomer and polymer, and preparation method thereof
The invention relates to a low-viscosity cyano resin and a preparation method thereof, belonging to the technical field of organic high polymer materials. The low-viscosity cyano resin is prepared by proportionally mixing benzoxazine-ring-containing tetra-ortho-phthalonitrile resin monomer and diluter with para-nonyl phenol-type phthalonitrile monomer. The benzoxazine-ring-containing tetra-ortho-phthalonitrile resin monomer and nonyl phenol-type phthalonitrile monomer can be blended within a wider proportion range; and the mixture has the advantages of lower and adjustable viscosity at low temperature, favorable flowability, low-temperature curing and other processing properties. Meanwhile, the addition of the para-nonyl phenol-type phthalonitrile monomer has small influence on the heat stability of the benzoxazine-ring-containing tetra-ortho-phthalonitrile resin. The mixed resin has favorable processability of the benzoxazine resin and excellent temperature tolerance of the cyano resin, has the advantages of low-temperature processing, medium-temperature molding and high-temperature application, and can be used in the fields of space navigation and ship structural materials, paints, adhesives, electronic packaging materials and the like. The preparation method is easy to control, has the advantages of low reaction temperature and energy saving, and is suitable for continuous industrial production.
Owner:725TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Bi-phthalonitrile resin glass fiber composite material toughened by poly(arylene ether nitrile) and preparation method thereof
InactiveCN101831173AImprove curing reaction rateImprove the problem of long curing timeSynthetic resin layered productsGlass/slag layered productsGlass fiberDecomposition
The invention provides a bi-phthalonitrile resin glass fiber composite material toughened by poly(arylene ether nitrile) and a preparation method thereof, belonging to the macromolecular material technical field. The glass fiber composite material comprises a single-layer or multilayer glass fiber cloth and a copolymer of poly(arylene ether nitrile) and bi-phthalonitrile resin distributed on the surface of the single-layer glass fiber cloth or among the multilayer glass fiber cloth, wherein, the mass ratio of the copolymer of the poly(arylene ether nitrile) and the bi-phthalonitrile resin to the glass fiber cloth is 4:6; and the mass ratio of the poly(arylene ether nitrile) and the bi-phthalonitrile resin is (2-6): (34-38). The preparation method comprises the following steps: fusing and mixing the bi-phthalonitrile resin and poly(arylene ether nitrile) powder, cooling and pulverizing into powder, spraying the powder on the surface of the glass fiber cloth, laminating, molding by compression, and finally carrying out thermal treatment to obtain the composite material. In the invention, the bending strength of the bi-phthalonitrile resin glass fiber composite material toughened by the poly(arylene ether nitrile) is 500-650MPa, and the initial decomposition temperature is above 450 DEG C; and the composite material can be widely applied to the high-tech fields such as aerospace composite materials, machinery, electronic engineering and the like.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
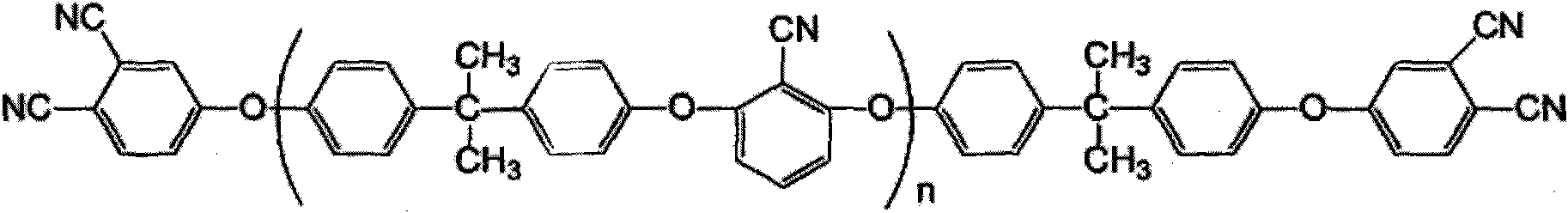
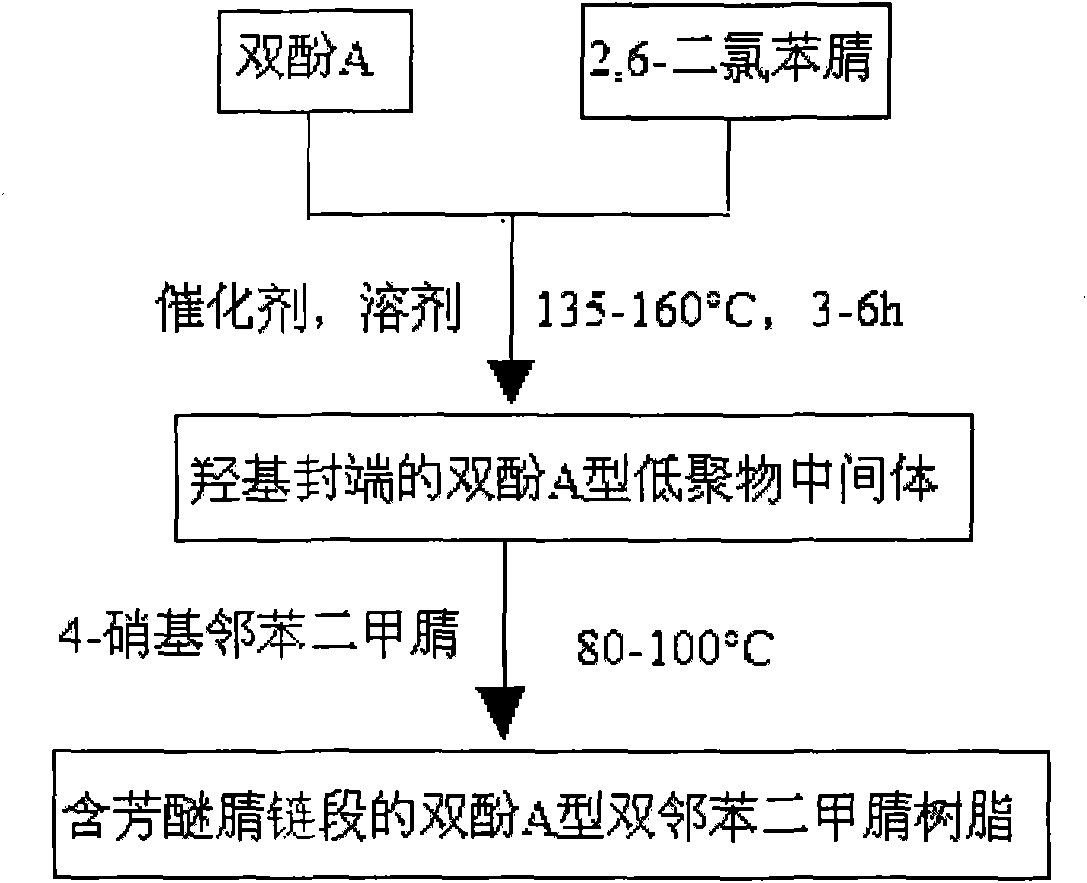
Bisphenol A type bis-phthalonitrile resin with aryl ether nitrile segments, cured product and preparation method thereof
InactiveCN101914038ALow water absorptionImprove curing effectCarboxylic acid nitrile preparationOrganic compound preparationPhthalonitrileSolvent
The invention discloses a bisphenol A type bis-phthalonitrile resin with aryl ether nitrile segments, a cured product and a preparation method thereof and belongs to the field of polymer materials. Raw materials of bisphenol A and 2,6-dichlorobenzonitrile are subjected to nucleophilic substitution reaction, anhydrous potassium carbonate and anhydrous sodium carbonate are used as a catalyst, a hydroxyl-terminated bisphenol A type oligomer intermediate is formed in mixed liquor of a strong polar solvent and toluene, and then hydroxyl groups are substituted by 4-nitrophthalonitrile, thus obtaining the resin. A cure-crosslinking agent, the mass of which is equal to 5 percent of the resin, is added into the resin, and then after pre-curing at 220-240DEG C and heat treatment at 375DEG C at least, the cured product of the resin can be obtained. The bis-phthalonitrile resin provided by the invention has different chain lengths and the aryl ether nitrile segments as well as a low melting point and wider curing process temperature, and the cured product is of a net structure which comprises a phthalocyanine ring and a triazine ring and has excellent heat stability. The manufacturing methods of the resin and the cured product are simple and controllable and are suitable for industrial production.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
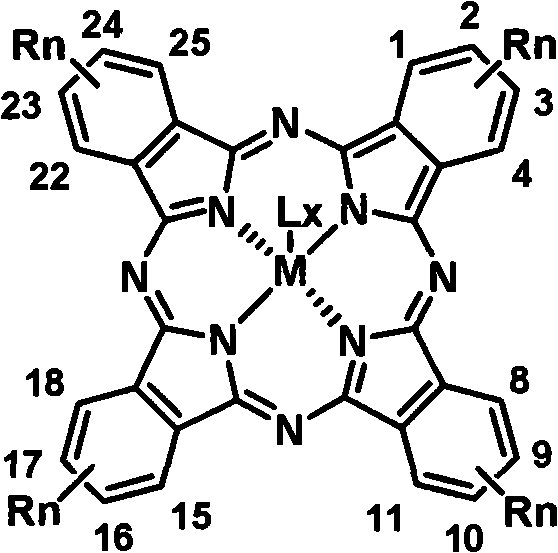
Soluble tetraalkyl phthalocyanine compound and preparation method thereof
The invention relates to soluble tetra-alkyl phthalocyanine compounds and a method for making the same. The soluble tetra-alkyl phthalocyanine compounds have the following general structure, wherein Rn represents straight-chain or branching chain alkane, n represents the number of straight-chain or branching chain alkane, M represents center ligand of bivalence or more, L represents axial ligand, X represents the number of L. the soluble tetra-alkyl phthalocyanine compounds uses 4-alkyl phthalonitrile as starting material, have good solubility in organic solvent, have processing characterics in solution, and have potential applications in photoelectron material and information technology fields.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
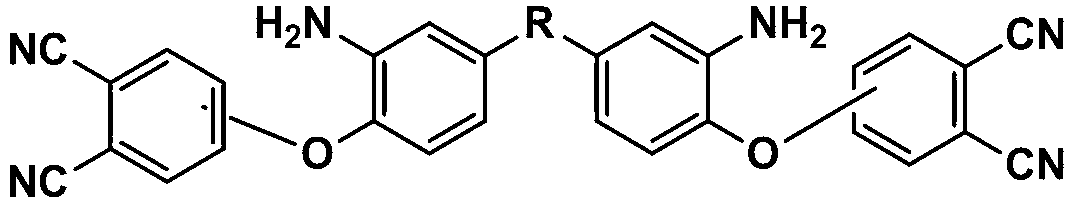
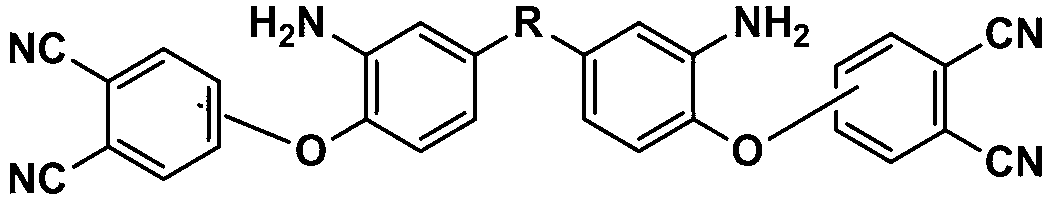
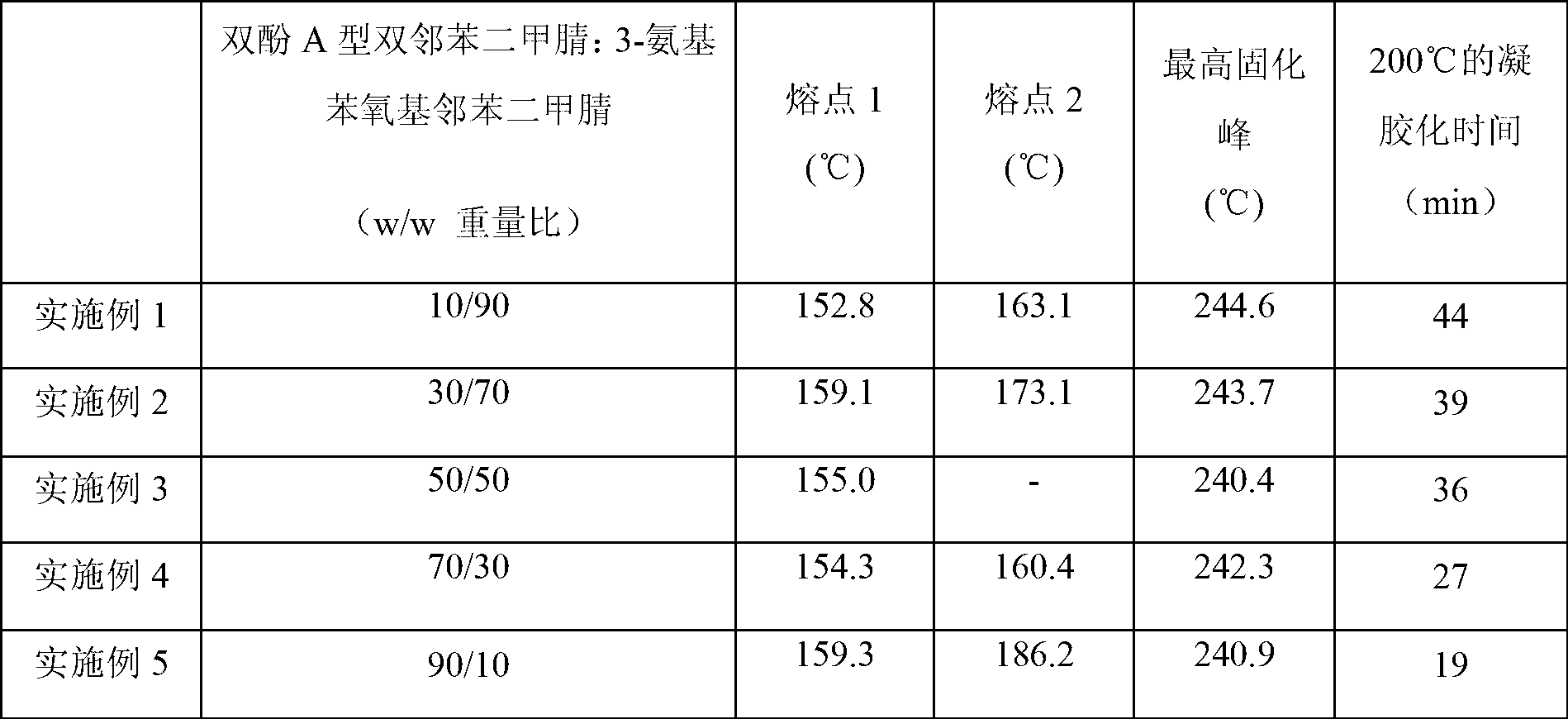
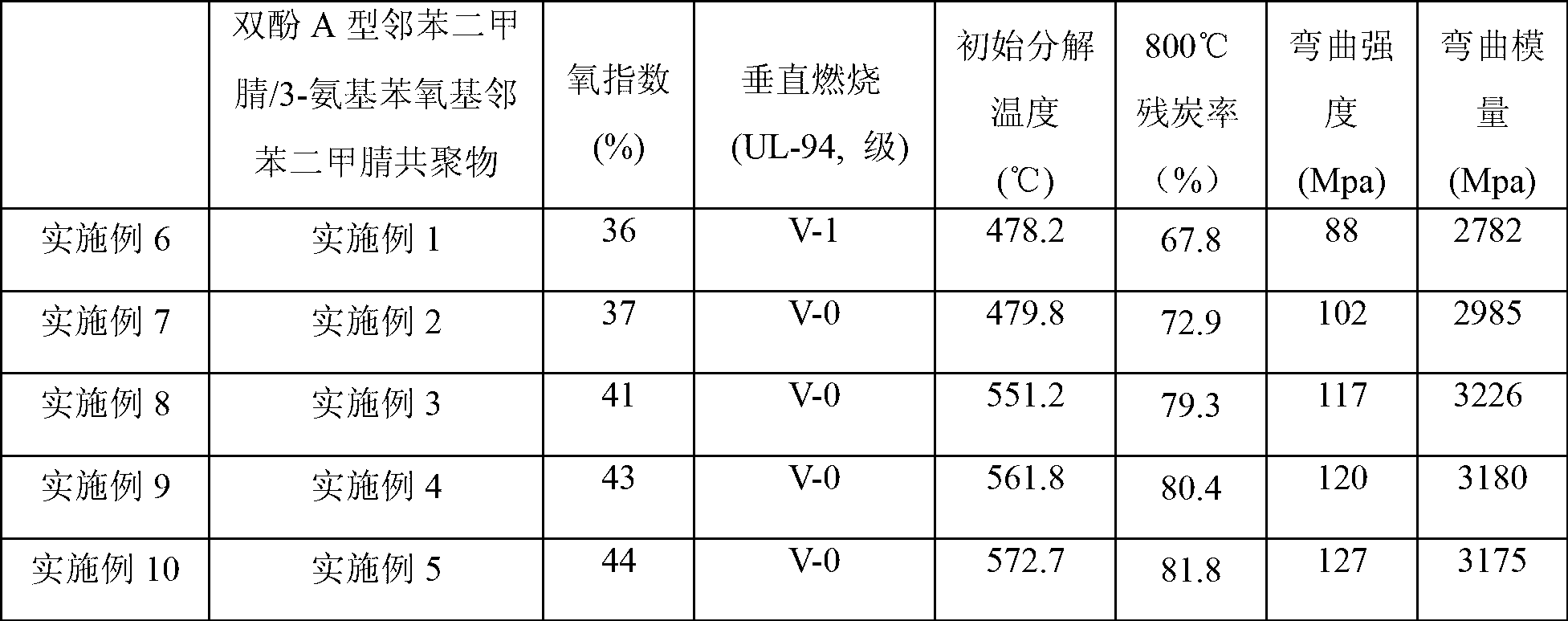
Bisphthalonitrile-amino phenoxy phthalonitrile copolymer and condensate, and glass fiber composite material and preparation method thereof
InactiveCN103012790AImprove flame retardant performanceImprove bending strengthGlass fiberPolymer science
The invention belongs to the technical field of high molecular materials, in particular relates to a bisphthalonitrile-amino phenoxy phthalonitrile copolymer and condensate as well as a glass fiber composite material and a preparation method thereof. The bisphthalonitrile-amino phenoxy phthalonitrile copolymer, provided by the invention, is obtained by fusing and copolymerizing bisphthalonitrile and amino phenoxy phthalonitrile at the temperature of 200-220 DEG C. The method adopts the amino phenoxy phthalonitrile with low cost to obtain the bisphthalonitrile-amino phenoxy phthalonitrile copolymer and the condensate; and the obtained condensate has excellent flame retardance (oxygen index is more than 36 percent); the bending strength achieves 88-130 MPa; the original decomposition temperature is above 478 DEG C; and the carbon residue rate at the temperature of 800 DEG C is more than 65 percent.
Owner:成都德美精英化工有限公司 +1
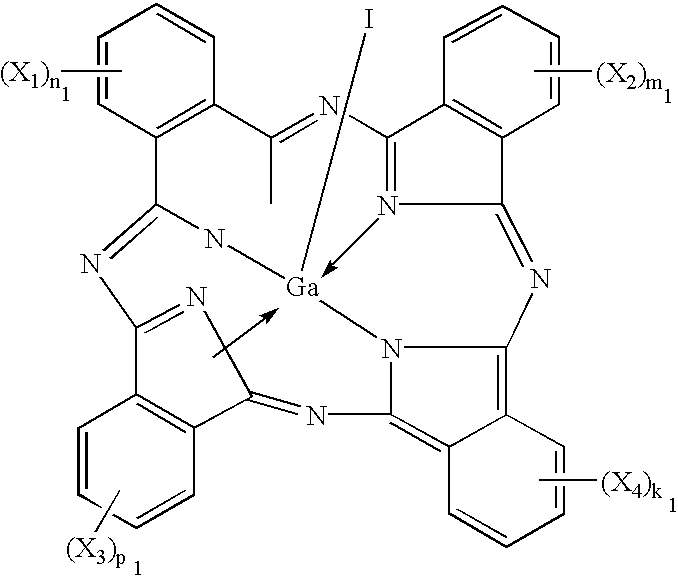
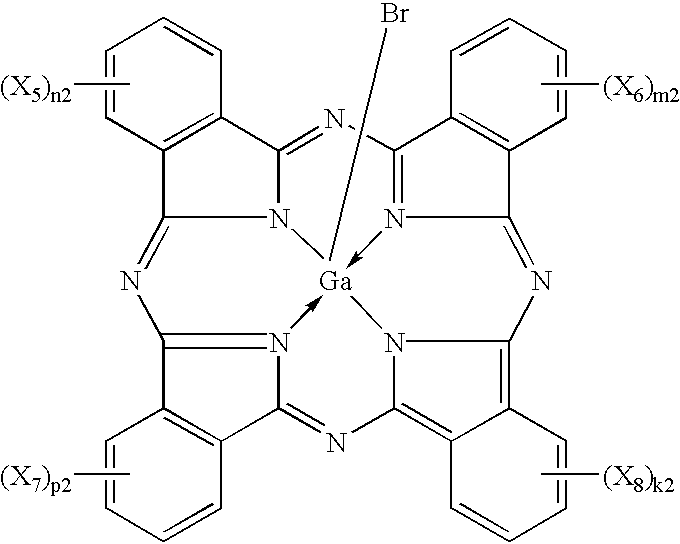
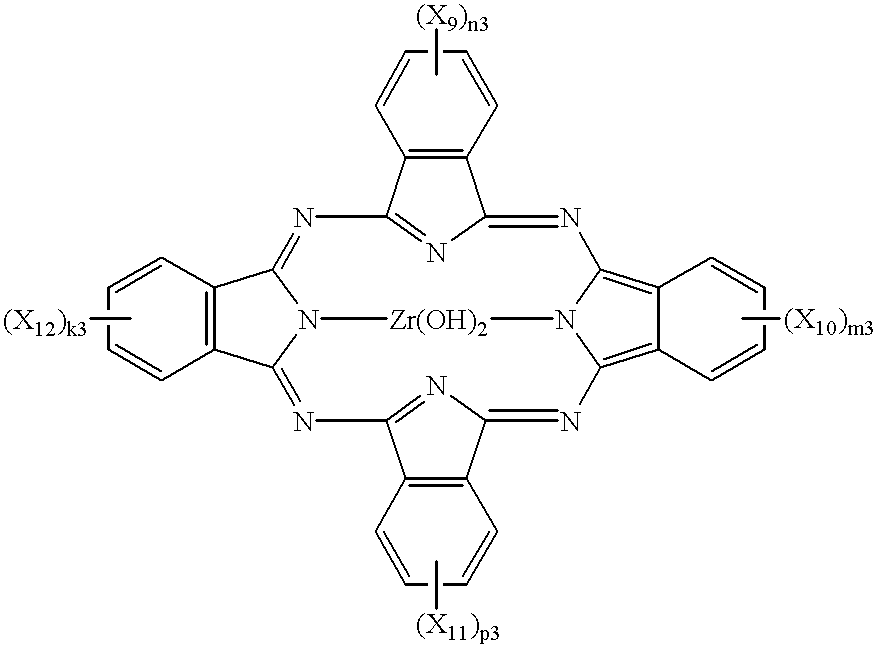
Phthalocyanine compounds, process for production thereof and electrophotographic photosensitive member using the compounds
InactiveUS6472524B2High sensitivityImprove potential stabilityOrganic chemistryPorphines/azaporphinesTriiodideX-ray
Iodogallium phthalocyanine and bromogallium phthalocyanine having novel crystalline forms characterized by X-ray diffraction patterns according to CuKalpha characteristic X-ray diffraction method and exhibiting excellent sensitivity can be obtained through appropriate selection of a reaction solvent, followed by milling or stirring in an appropriate solvent. For example, alpha-chloronaphthalene is a suitable solvent for reaction between phthalonitrile and gallium triiodide or tribromide to provide iodogallium phthalocyanine or bromogallium phthalocyanine. Reaction of chlorogallium phthalocyanine or hydroxygallium phthalocyanine with hydroiodic (or hydrobromic) acid is also effective for providing a novel crystal form of iodo- (or bromo-) gallium phthalocyanine. Zirconium phthalocyanine exhibiting good electrophotographic performances can be obtained through a similar process.
Owner:CANON KK
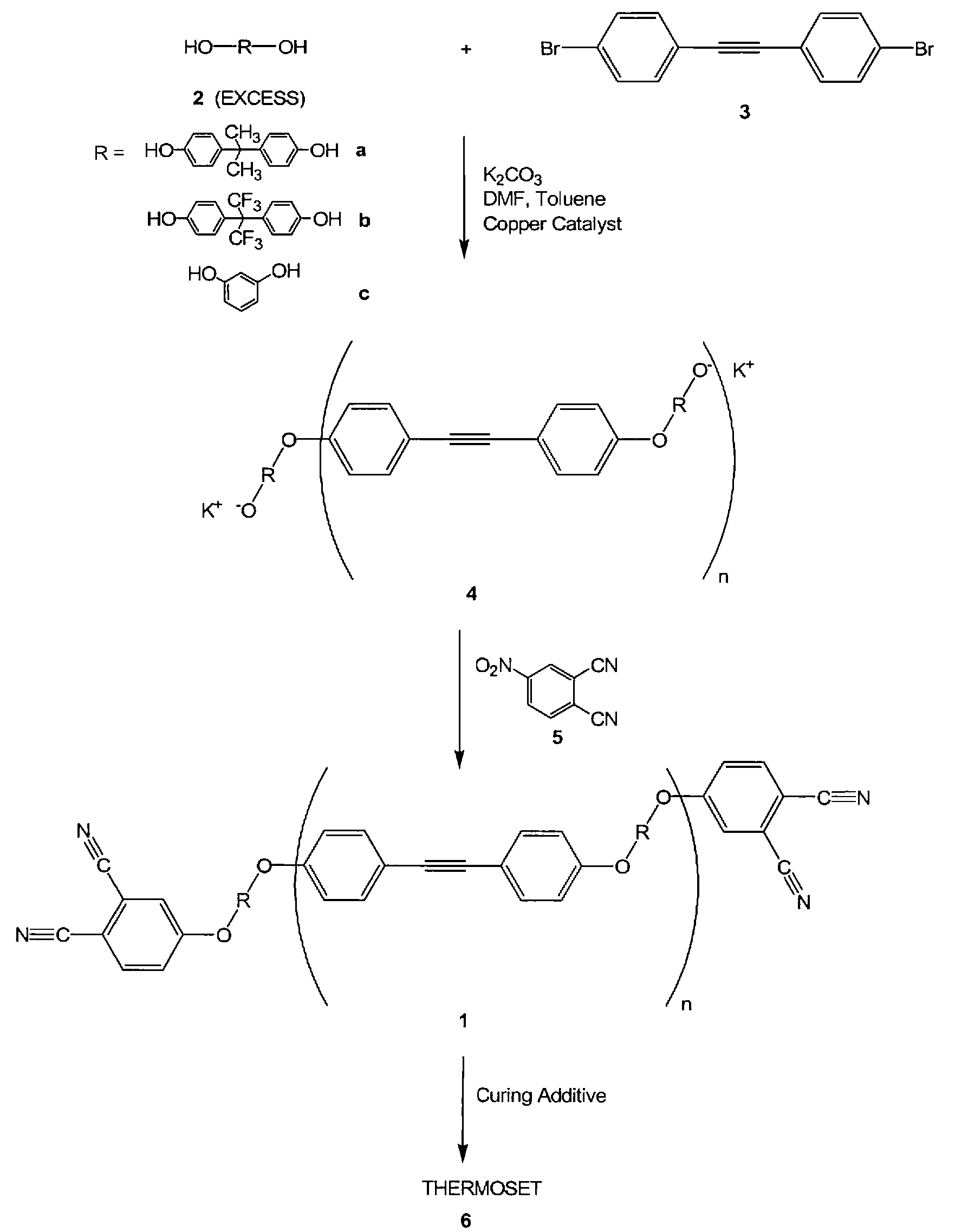
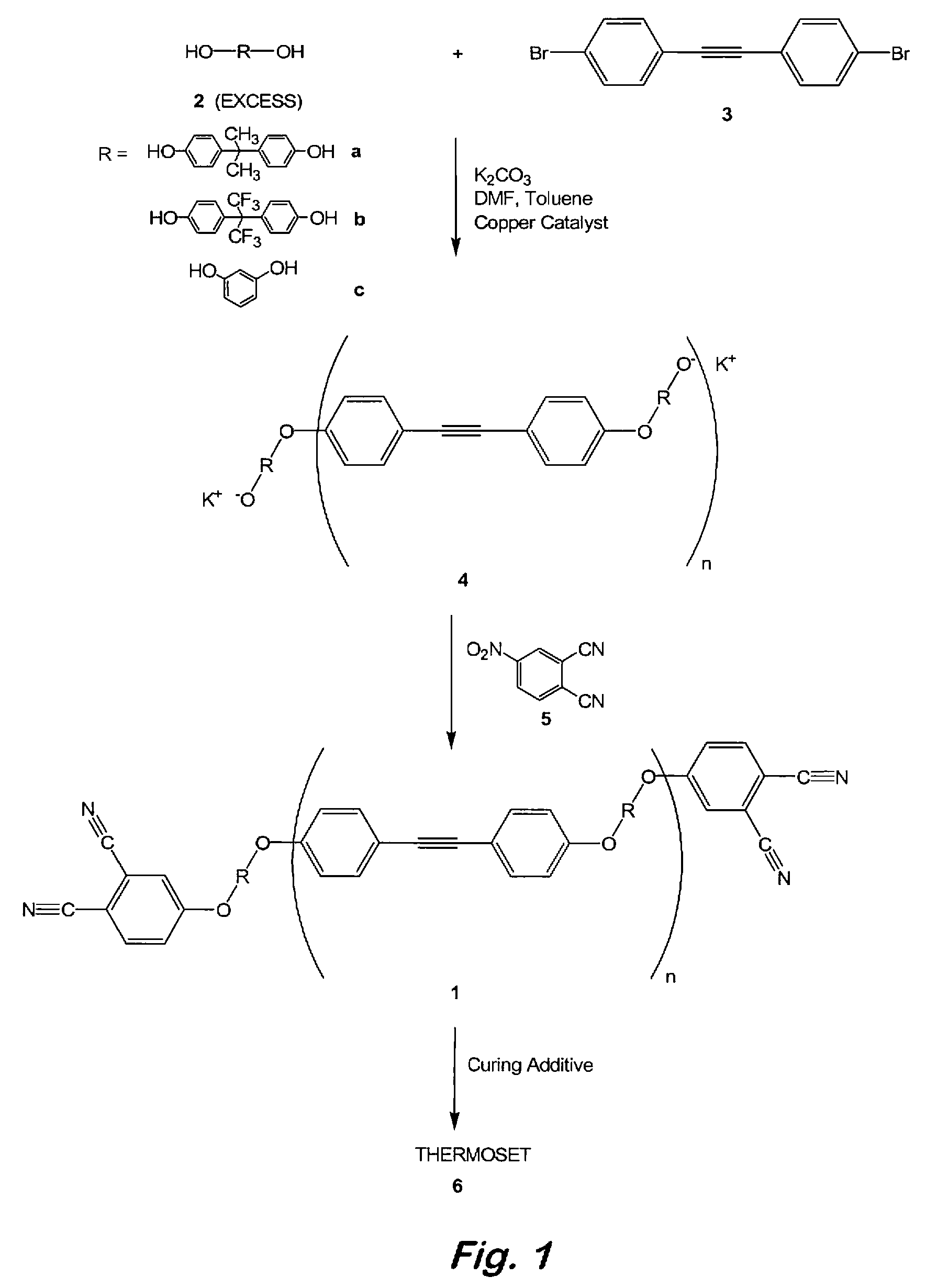
Oligomeric hydroxy arylether phthalonitiles and synthesis thereof
An aromatic ether oligomer or polyaromatic ether comprising the formula: wherein Ar is an independently selected divalent aromatic radical; formed by reacting a dihydroxyaromatic with a dihaloaromatic; and wherein the reaction is performed in the presence of a copper compound and cesium carbonate. The polyaromatic ether is formed when neither the dihydroxyaromatic nor the dihaloaromatic is present in an excess amount. The aromatic ether oligomer is formed by using an excess of either dihydroxyaromatic or dihaloaromatic. A phthalonitrile monomer comprising the formula: formed by reacting a 3- or 4-nitrophthalonitrile with a hydroxy-terminated aromatic ether oligomer. A thermoset formed by curing the phthalonitrile monomer. Processes for forming all the above.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Polyhaloacridones compound, intermediate and synthetic method thereof
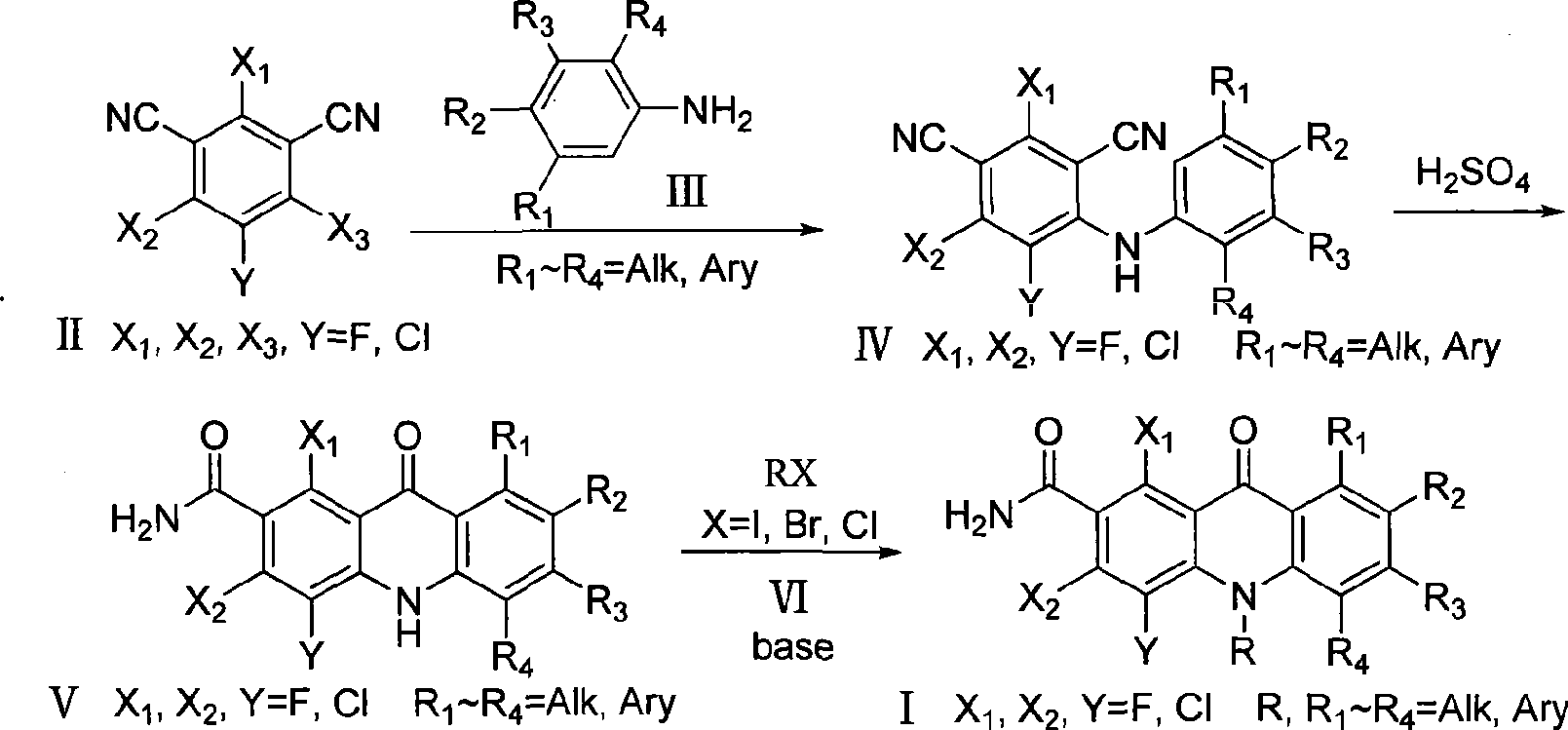
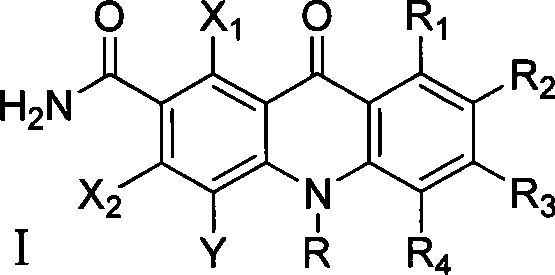
The invention discloses multi-halogenated acridone compounds, intermediates and a synthetic method thereof, wherein, the synthetic method comprises the steps: under the condition of alkali or no alkali, multi-halogenated m-phthalonitrile with the structural formula of (II) and phenylamine compound with the structural formula of (III) are reacted in a non-protonic solvent at certain temperature ( 0 to 90 DEG C ), thus generating the intermediate with the structural formula of (IV); then the intermediate (IV) is reacted with sulphuric acid to synthetize the multi-halogenated acridone compound with the structural formula of (V), and then the formula (V) is treated with alkylation or arylation reaction of nitrogen atoms to synthetize the multi-halogenated acridone compound (I).
Owner:YUNNAN UNIV
Phthalonitrile-terminated polyimide resin containing phthalazinone structure, cured product and preparation method thereof
The invention belongs to the field of synthesis of polymer materials and specifically relates to phthalonitrile-terminated polyimide resin containing a phthalazinone structure, a cured product and a preparation method thereof. The phthalonitrile-terminated polyimide resin containing the phthalazinone structure is prepared by taking 4-(3-aminophenoxy) phthalonitrile as a terminating agent and carrying out solution nucleophilic substitution reaction. The method has simple steps and is convenient and feasible. The polyimide resin shows great solubility in commonly used polar solvents, can be processed and molded by a variety of ways, and simultaneously has great curing reaction activity. The cured product of the polyimide resin containing the phthalazinone structure with dimension stability and high thermal stability can be obtained by carrying out pre-curing on the resin at the temperature of 150-300 DEG C and carrying out thermal treatment at the temperature of 350-400 DEG C in the presence of aromatic diamine. The phthalonitrile-terminated polyimide resin containing the phthalazinone structure can be used for preparing coatings, insulating paint, sizing agents, thin films, high-performance composite materials and the like and has wide application prospects.
Owner:DALIAN UNIV OF TECH
Synthesis method of phthalonitrile and arylacetylene-terminated aromatic imide
ActiveCN104130177AImprove the disadvantages of difficult processingEasy to processOrganic chemistryAcetic anhydrideSynthesis methods
The invention discloses a synthesis method of phthalonitrile and arylacetylene-terminated aromatic imide. The method comprises the following steps: I, synthesis of a single-component system: reacting tetracid dianhydride serving as a raw material with a raw material containing amine phthalonitrile in a polar solvent to obtain an intermediate 1, adding acetic anhydride to obtain an intermediate 2, and reacting the intermediate 2 with a raw material containing amine arylacetylene to obtain a target product; and II, synthesis of a multi-component system: reacting the tetracid dianhydride serving as the raw material with the raw material containing the amine phthalonitrile to obtain a first monomer; reacting the raw material containing the amine arylacetylene with the tetracid dianhydride serving as the raw material to obtain a second monomer; and performing melt blending or solution blending on the first monomer and the second monomer to obtain a target product. By adopting the synthesis method, the aromatic polyimide, a polyphthalonitrile resin and a polyarylacetylene resin are introduced into the same curing system through physical blending or chemical modification, so that the processing performance of the product can be improved effectively, and the processing cost is lowered.
Owner:SICHUAN UNIV
Environmentally friendly willow woven product water-based paint
The present invention discloses an environmentally friendly willow woven product water-based paint, and relates to the technical field of willow woven product water-based paints. The willow woven product water-based paint is made from the following materials by mass: 15-20% of methyl methacrylate, 3-5% of acrylic acid, 12- 16% of styrene, 10-15% of butyl acrylate, 0.5-1% of dibenzoyl peroxide, 0.2-0.5% of beta-thionaphthol, 4 -8% of 1-isopropoxy-2-propanol, 5-9% of diisopropanolamine, 0.5-1% of dioctyl phthalate, 3-5% of propylene glycol phenyl ether, 0.2-0.5% of Agitan 315, 0.1-0.2% of 2,4,5,6 -tetrachloro phthalonitrile, 0.8-1.2% of a polyurethane thickener, 1-3% of polyvinyl alcohol, 0.1-0 .3% of naphthenate, 0.5-1.5% of a dispersant, 0.2-0.5% of a leveling agent, 0.5-1% of an adhesion promoter, and 40-45% of water. The environmentally friendly willow woven product water-based paint may be applied to different willow woven product surfaces, and is delicate and fine in paint appearance, anti-mildew, anti-algae, strong in hiding power, and free of environment pollution.
Owner:赛诺(浙江)聚氨酯新材料有限公司
Preparation method of cross-linked poly(arylene ether nitrile) dielectric film with high temperature resistance
The invention relates to a synthesis method for cross-linked poly(arylene ether nitrile) and a preparation method of a high-temperature-resistant dielectric film thereof, belonging to the field of special high-polymer materials. Starting from the main molecular structure design of poly(arylene ether nitrile), hydroxyl-terminated poly(arylene ether nitrile) is synthesized by using hydroquinone withan amino-containing side chain as one of reaction monomers, and then phthalonitrile is introduced to the tail end of a molecular main chain to synthesize the cross-linkable poly(arylene ether nitrile). A cross-linked poly(arylene ether nitrile) film can be obtained by adopting curtain-coating film forming and low-temperature self-cross-linking strategies. The obtained film has excellent thermal properties, mechanical properties and dielectric properties, and can be used as a high-temperature-resistant dielectric film. The preparation method disclosed by the invention is simple in process andeasy for industrial operation, and the application range of the poly(arylene ether nitrile) can be widened.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Phthaphthalonitrile resin prepreg, composite material and preparation method of composite material
ActiveCN108454135AGood mechanical propertiesImprove molding qualityPhthalonitrileTemperature resistance
Owner:AEROSPACE RES INST OF MATERIAL & PROCESSING TECH +1
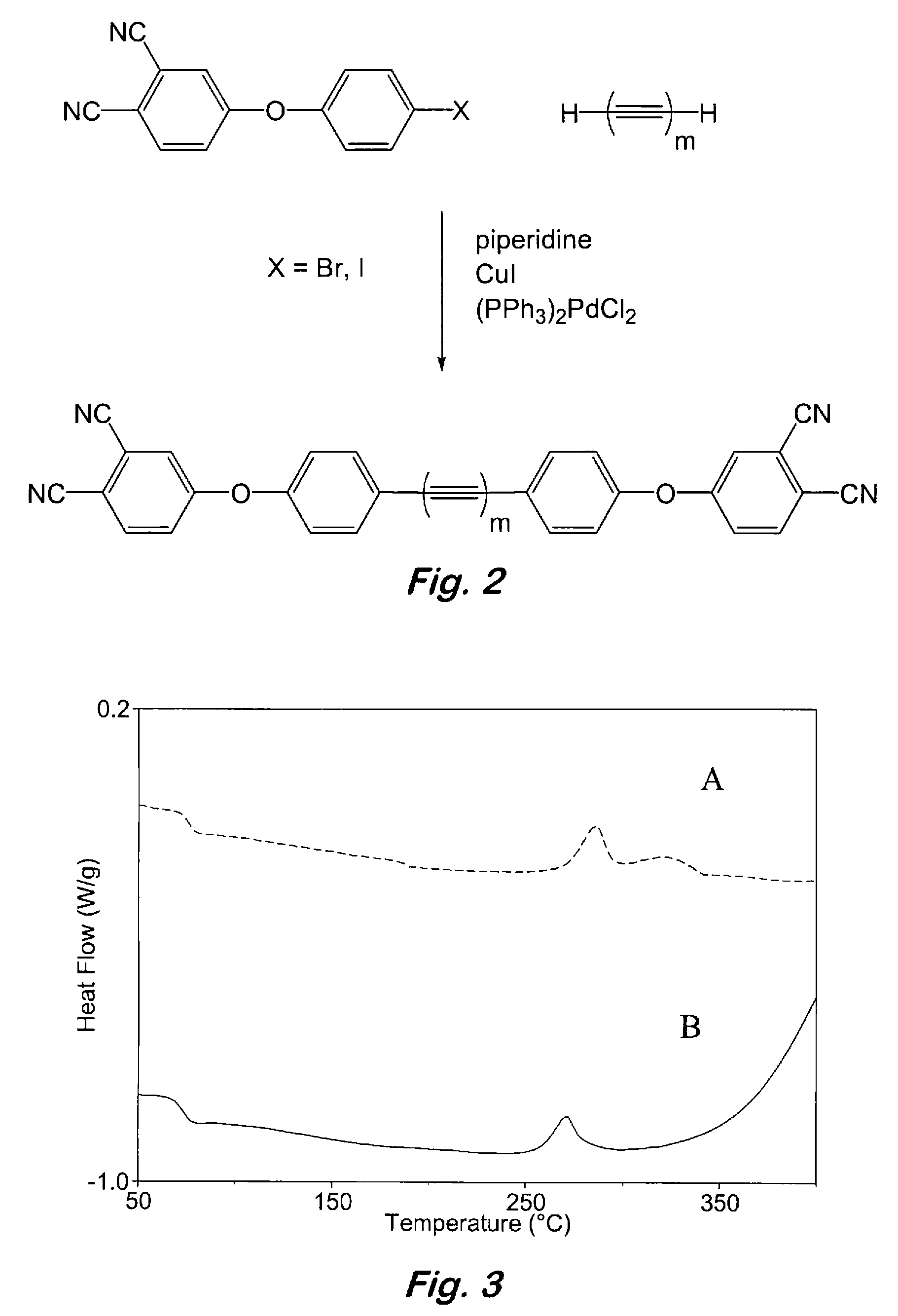
Aromatic ether and alkynyl containing phthalonitriles
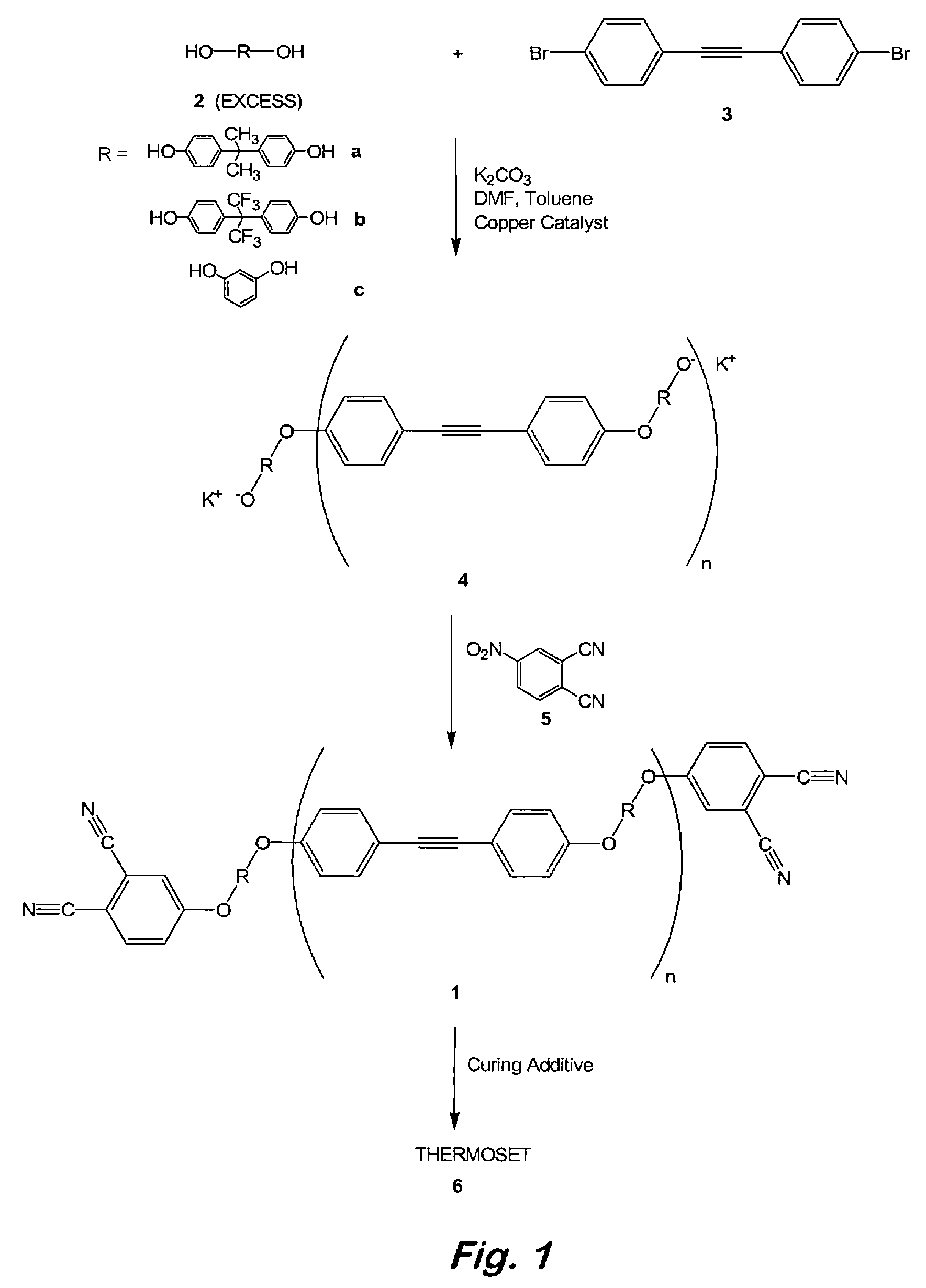
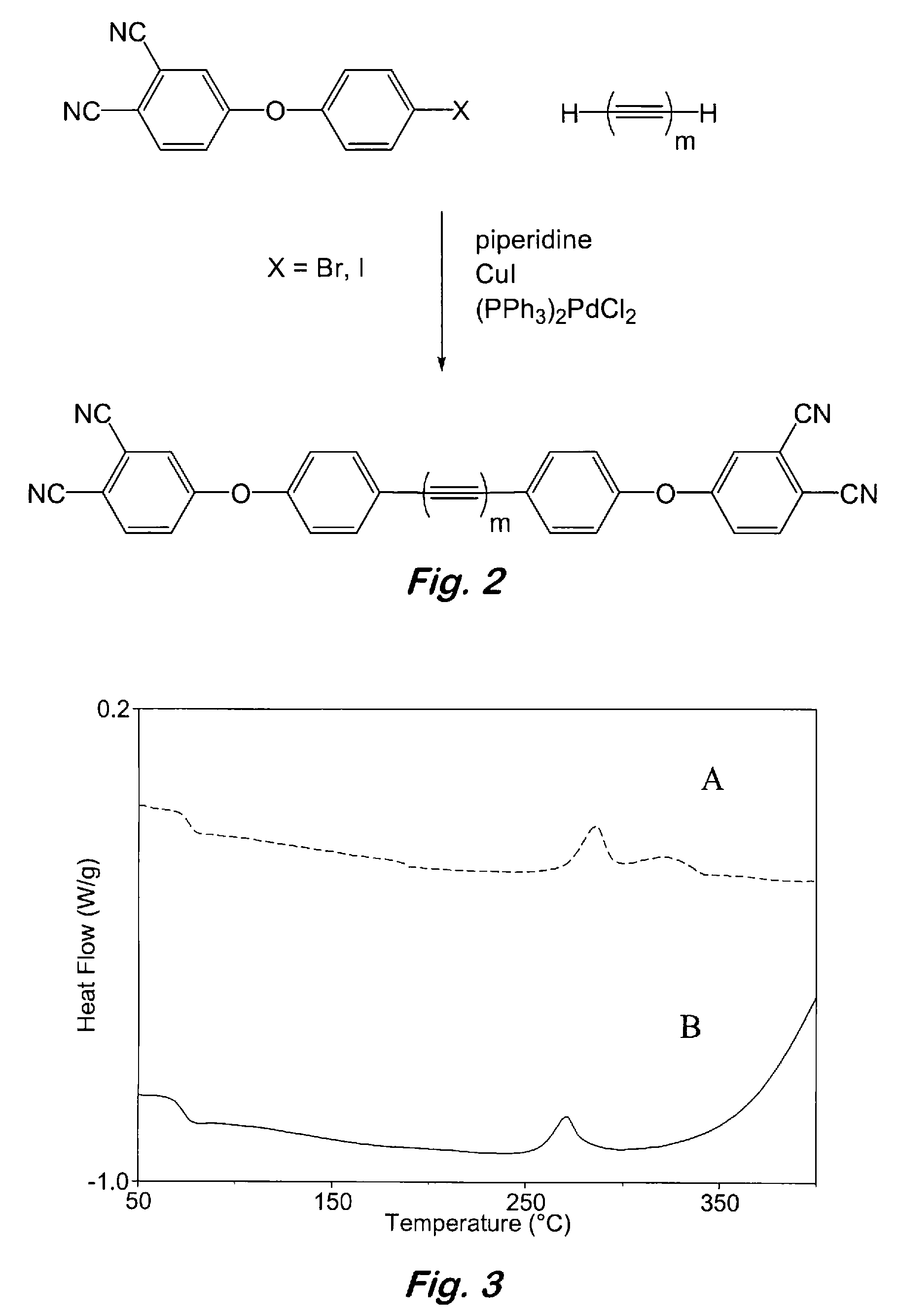
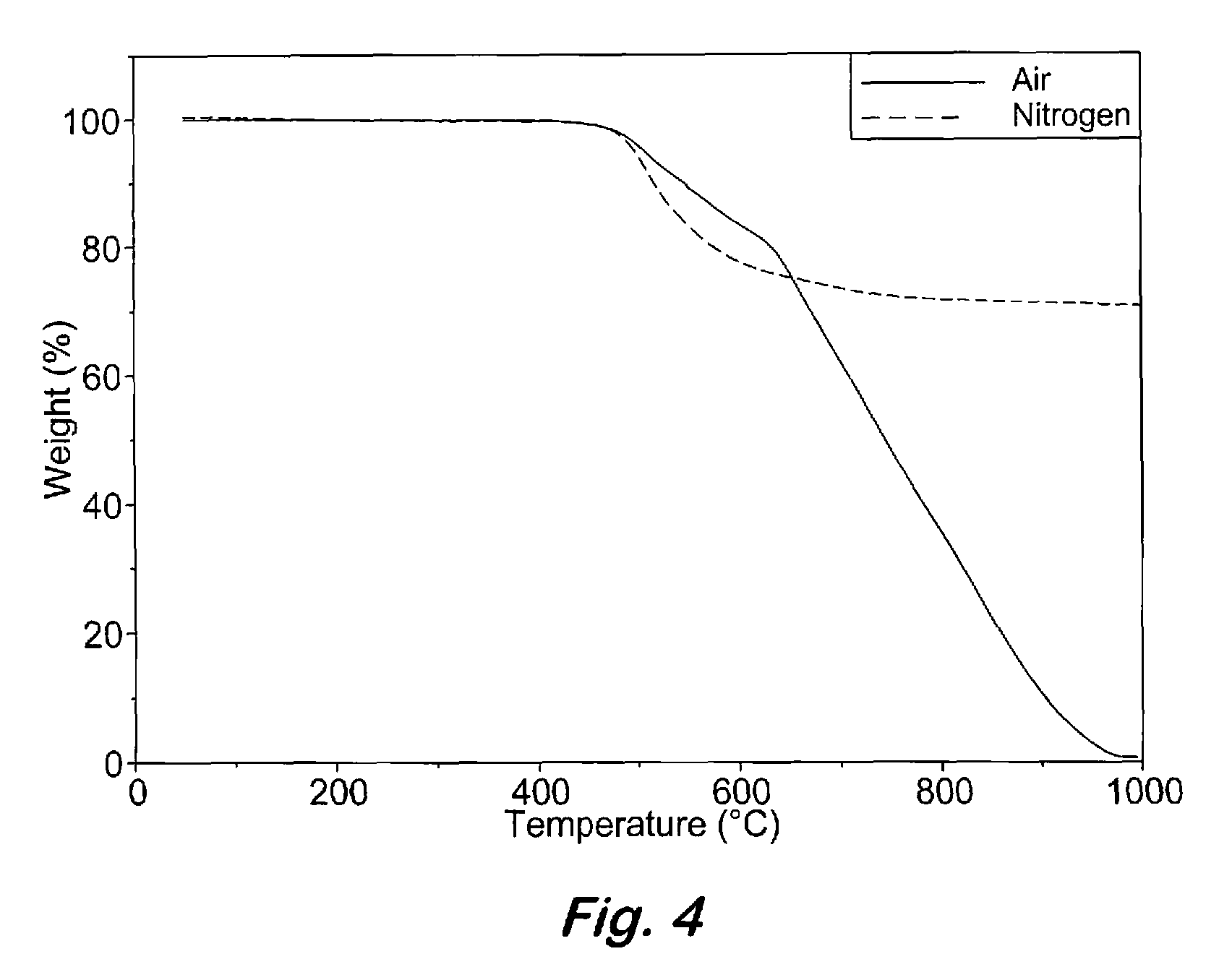
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
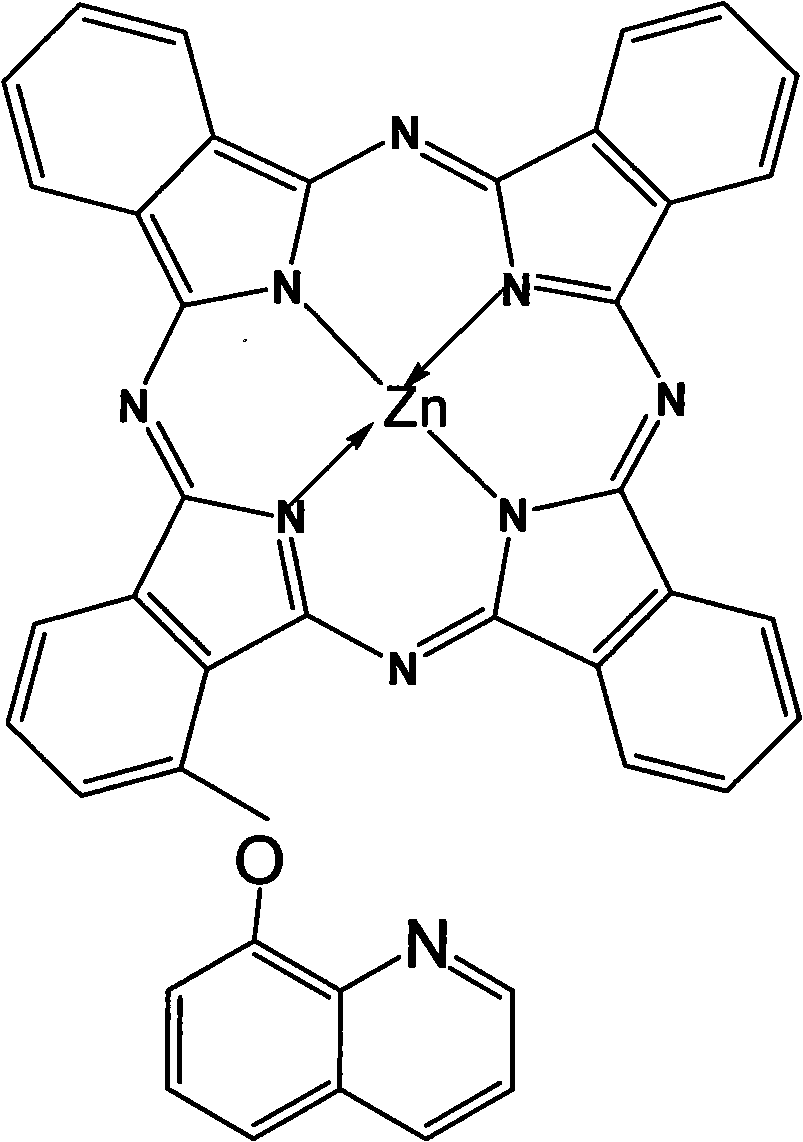
Alpha-(8-quinolineoxy)mono-substituted zinc phthalocyanine and preparation method thereof
ActiveCN101260110ASingle structureEasy to purifyZinc organic compoundsCoordination geometryZinc phthalocyanine
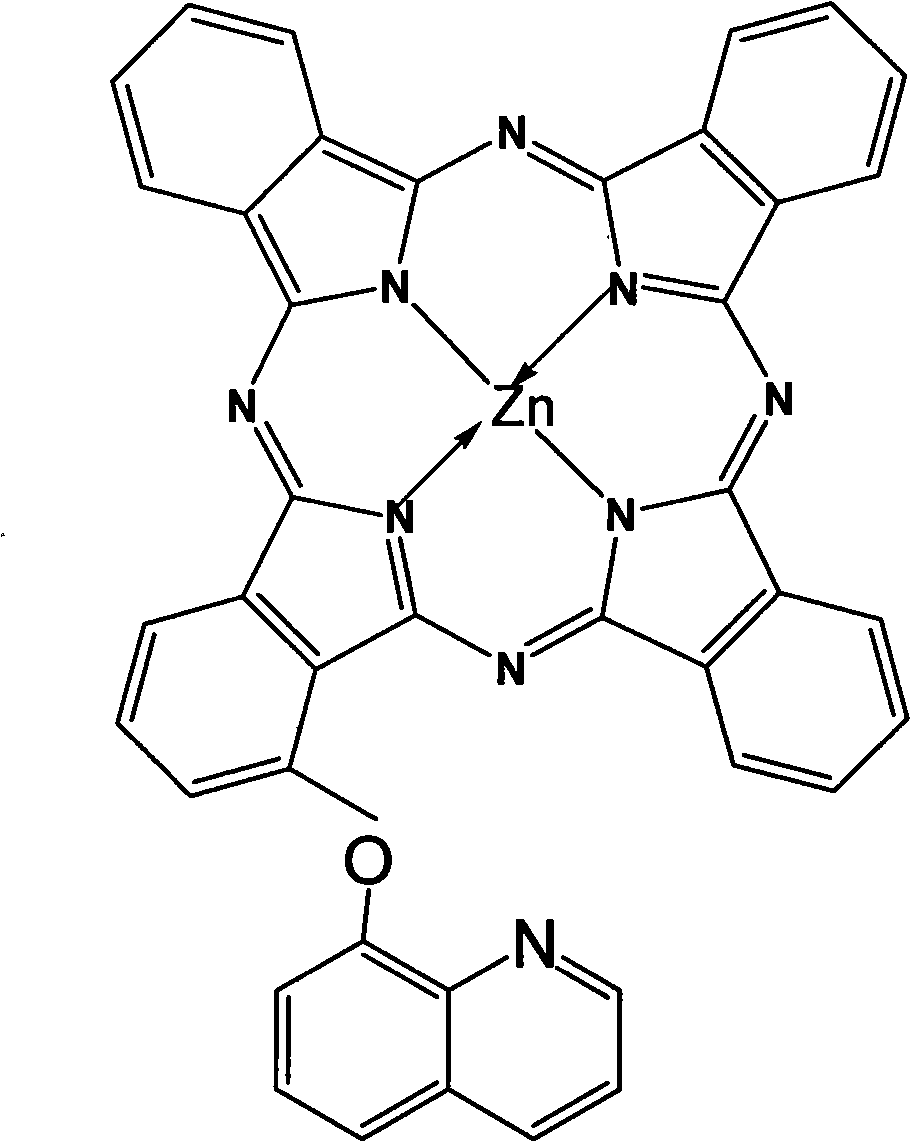
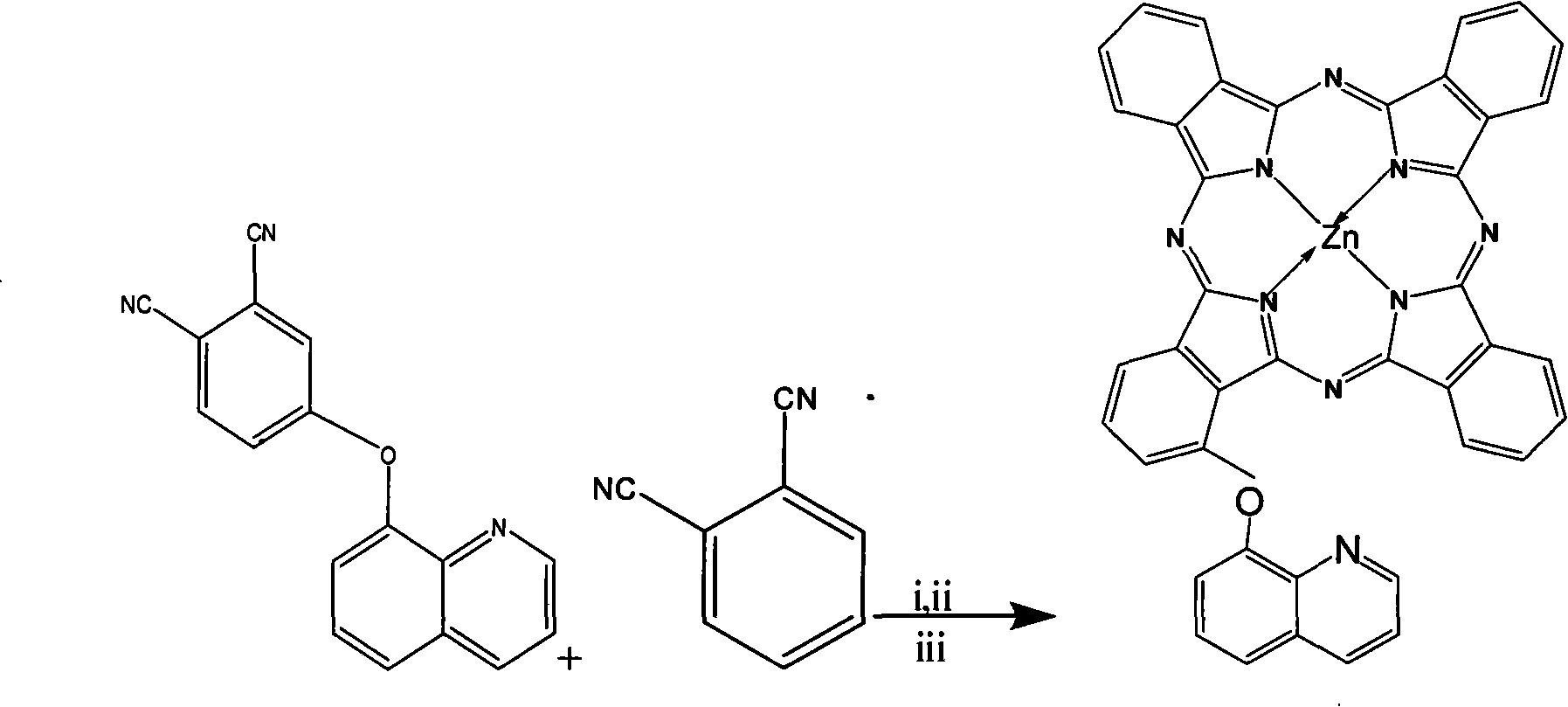
The invention provided an alpha-(8-quinoly) single substituted zinc phthalocyanine and a preparation method thereof, with the molecular formula of C41H21N9OZn; the preparation method is as follows: 8-hydroxyl quinoly and 3- nitrophthalonitrile are adopted as the original substances to synthesize 3-(8- quinoly) phthalonitrile; then 3-(8- quinoly) phthalonitrile, phthalonitrile, and the corresponding zinc salt are adopted as the original substances to synthesize a coordination compound of Phthalocyanine-zinc under the DBU catalysis, and then the coordination compound of Phthalocyanine-zinc is separated to obtain the final product. The coordination compound provided by the invention has a single structure without any isomer, and is characterized in defined structure and easy separation. The coordination compound can be applied in the high-tech fields such as the preparation of novel anti-cancer drugs.
Owner:FUZHOU UNIV
Aromatic ether and alkynyl containing phthalonitriles
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com