Preparation method of cross-linked poly(arylene ether nitrile) dielectric film with high temperature resistance
A technology of polyarylether nitrile and cross-linking type, which is applied in the field of synthesis of cross-linked polyarylether nitrile, which can solve the problems of metal salts affecting product performance, poor catalyst dispersion, and reducing resin performance, etc., and achieve excellent thermal performance. Performance, excellent heat resistance and mechanical properties, performance-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
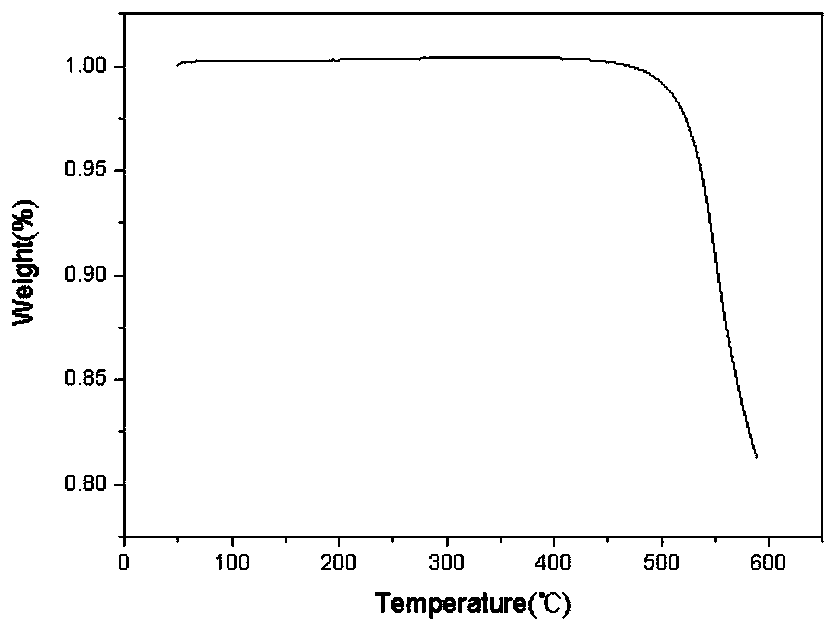
Image
Examples
experiment example 1
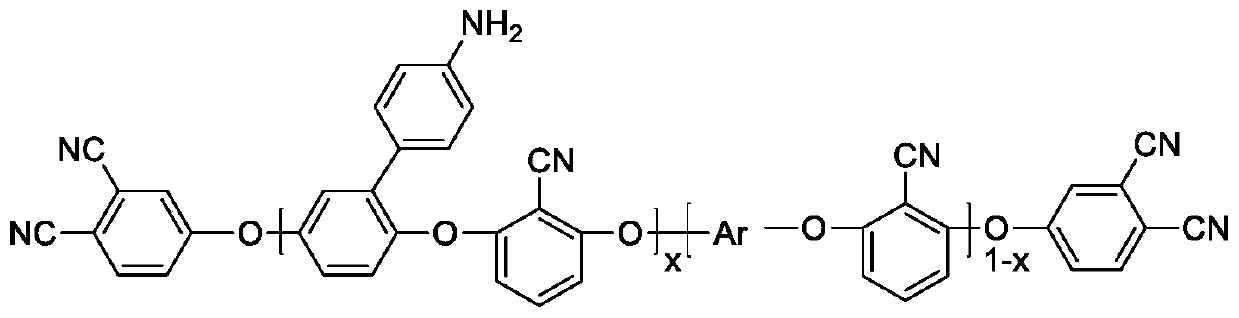
[0030] Biphenyl-type polyarylether nitrile end-capped with phthalonitrile containing amino groups in the side chain:
[0031] (1) Add potassium carbonate (0.12mol), biphenol (0.0918mol), 4-aminophenylhydroquinone (0.0102mol) to a reaction flask containing 50ml of dehydrating agent toluene and 75ml of N-methylpyrrolidone , heat the reaction at 130°C to reflux for 2 hours, then remove the water generated by the reaction, and control the reaction temperature between 130-140°C;
[0032] (2) Add 2,6-dichlorobenzonitrile (0.1mol) to the system, keep the reaction between 130-140°C for 1h, then slowly raise the temperature, raise the temperature to 170°C in about 2h, maintain the reaction at 170°C for 2h, react The system reaches the maximum viscosity;
[0033] (3) Lower the system temperature to 100°C, add 4-nitrophthalonitrile (0.02mol), potassium carbonate (0.02mol) and N-methylpyrrolidone (30ml), and then continue the reaction for 4h;
[0034] (4) Pour the obtained crude product...
experiment example 2
[0040] Bisphenol A type polyarylether nitrile end-capped by phthalonitrile containing amino groups in the side chain:
[0041] (1) Add potassium carbonate (0.12mol), bisphenol A (0.0918mol), and 4-aminophenylhydroquinone (0.0102mol) to a reaction flask containing 50ml of dehydrating agent toluene and 75ml of N-methylpyrrolidone , heating the reaction at 130°C to reflux for 2 hours, then removing the water generated by the reaction, and controlling the reaction temperature between 130-140°C;
[0042] (2) Add 2,6-dichlorobenzonitrile (0.1mol) to the system, keep the reaction between 130-140°C for 1h, then slowly raise the temperature, raise the temperature to 170°C in about 2h, maintain the reaction at 170°C for 2h, react The system reaches the maximum viscosity;
[0043] (3) Lower the system temperature to 100°C, add 4-nitrophthalonitrile (0.02mol), potassium carbonate (0.02mol) and N-methylpyrrolidone (20ml), and continue the reaction for 3h;
[0044] (4) Pour the obtained c...
experiment example 3
[0050] Side chain amino-containing phthalonitrile-terminated bisphenol AF type polyarylether nitrile:
[0051] (1) Add potassium carbonate (0.12mol), bisphenol AF (0.0918mol), 4-aminophenylhydroquinone (0.0102mol) to a reaction flask containing 50ml of dehydrating agent toluene and 75ml of N-methylpyrrolidone , heating the reaction at 130°C to reflux for 2 hours, then removing the water generated by the reaction, and controlling the reaction temperature between 130-140°C;
[0052] (2) Add 2,6-dichlorobenzonitrile (0.1mol) to the system, keep the reaction between 130-140°C for 1h, then slowly raise the temperature, raise the temperature to 170°C in about 2h, maintain the reaction at 170°C for 2h, react The system reaches the maximum viscosity;
[0053] (3) Lower the temperature of the system to 100°C, add 4-nitrophthalonitrile (0.02mol), potassium carbonate (0.02mol) and N-methylpyrrolidone (20ml), and then continue the reaction for 4h;
[0054] (4) Pour the obtained crude pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com