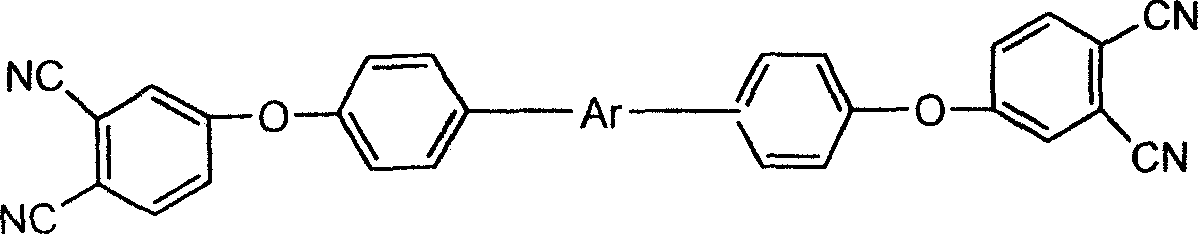Double end group ortho-benzene dimethyl nitrile-benzo oxazine resin, solidified substance and its preparing method and use
A technology based on phthalonitrile and nitrophthalonitrile, applied in the field of polymer synthetic materials, can solve the problems of benzoxazine resin, low curing processing temperature, and low initial decomposition temperature, etc. Achieve good curing reactivity, good mechanical strength and low water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
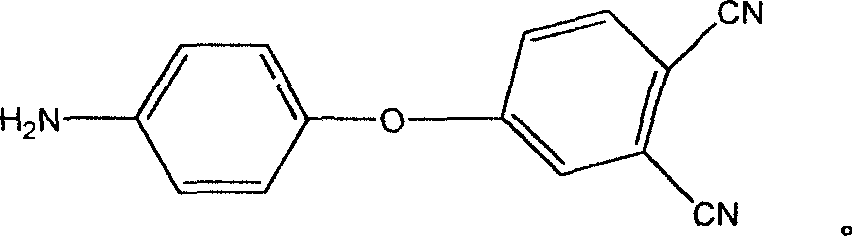
[0043] Preparation of 4-aminophenoxy-phthalonitrile monomer:
[0044] Add 4-nitrophthalonitrile, p-aminophenol, catalyst, and solvent into a four-neck flask, blow in nitrogen to replace the air in it, raise the temperature, and react at 80-90°C for 4-8 hours to stop the reaction , the reaction mixture was poured into 0.1M NaOH aqueous solution to precipitate, filtered, washed with water five times, and dried in a vacuum oven at 80°C for 24 hours to obtain 4-aminophenoxy-phthalonitrile monomer.
[0045] Preparation of double-ended phthalonitrile-benzoxazine resin:
[0046] Add dry 4-aminophenoxy-phthalonitrile monomer, aromatic dihydric phenol, and solvent into a four-necked bottle, pass nitrogen to replace the air in it, and pass nitrogen to replace the air in the reaction system, Then add paraformaldehyde in stages, the temperature should be controlled at about 60°C when adding, after adding, slowly raise the temperature to 70-120°C and continue to react for 3-5 hours; add h...
Embodiment 1
[0057] Embodiment 1 Preparation of double-terminated phthalonitrile-benzoxazine resin and cured product of the present invention
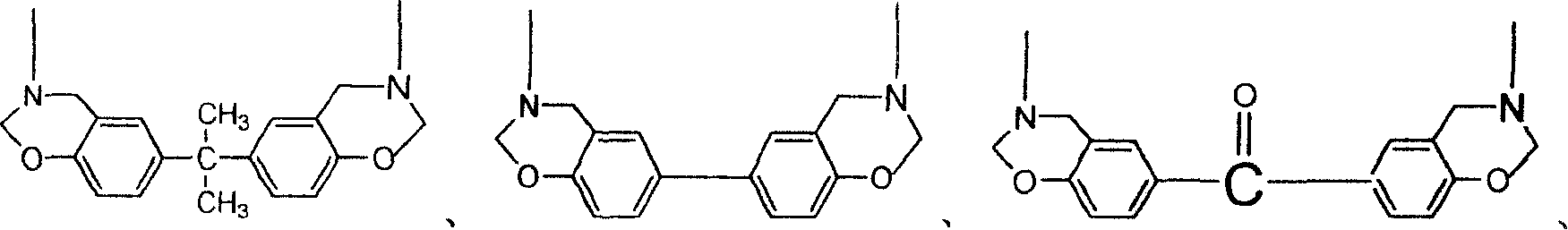
[0058] In this example, a phenol A type double-terminated phthalonitrile-benzoxazine resin and its cured product were prepared.
[0059] (1) Synthesis of 4-aminophenoxy group-phthalonitrile: the raw material formula component is (by mole):
[0060] 4-Nitrophthalonitrile 1 mole
[0061] p-Aminophenol 1 mole
[0062] Anhydrous Potassium Carbonate 1.1mole
[0063] Dimethylsulfoxide 15mole
[0064] (2) Synthesis of bisphenol A type double-terminated phthalonitrile-benzoxazine resin: the raw material formula components are (in molar parts):
[0065] 4-Aminophenoxy-phthalonitrile 2mole
[0066] Bisphenol A 1 mole
[0067] Paraformaldehyde 4mole
[0068] 1,4-dioxane / toluene (molar ratio 3:1) 15mole
[0069] (3) Preparation and detection of bisphenol A double-terminated phthalonitrile-benzoxazine resin and its cured product:
[0070] Add 4-nitroph...
Embodiment 2
[0073] Example 2 Preparation of double-terminated phthalonitrile-benzoxazine resin and cured product of the present invention
[0074] In this example, a biphenyl-type double-terminated phthalonitrile-benzoxazine resin and its cured product were prepared.
[0075] (1) Synthesis of 4-aminophenoxy group-phthalonitrile: starting material formula component is (by mole):
[0076] 4-Nitrophthalonitrile 1 mole
[0077] p-Aminophenol 1 mole
[0078] Anhydrous sodium carbonate 1.1mole
[0079] Dimethylformyl 20mole
[0080] (2) Synthesis of biphenyl-diphenol type double-terminated phthalonitrile-benzoxazine resin: the starting material formula components are (by moles):
[0081] 4-Aminophenoxy-phthalonitrile 2mole
[0082] Biphenol 1mole
[0083] Paraformaldehyde 4mole
[0084] 1,4-dioxane / toluene (molar ratio 3:1) 15mole
[0085] (3) Preparation and detection of biphenol-type double-terminated phthalonitrile-benzoxazine resin and its cured product
[0086]Add 4-nitrophthaloni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com