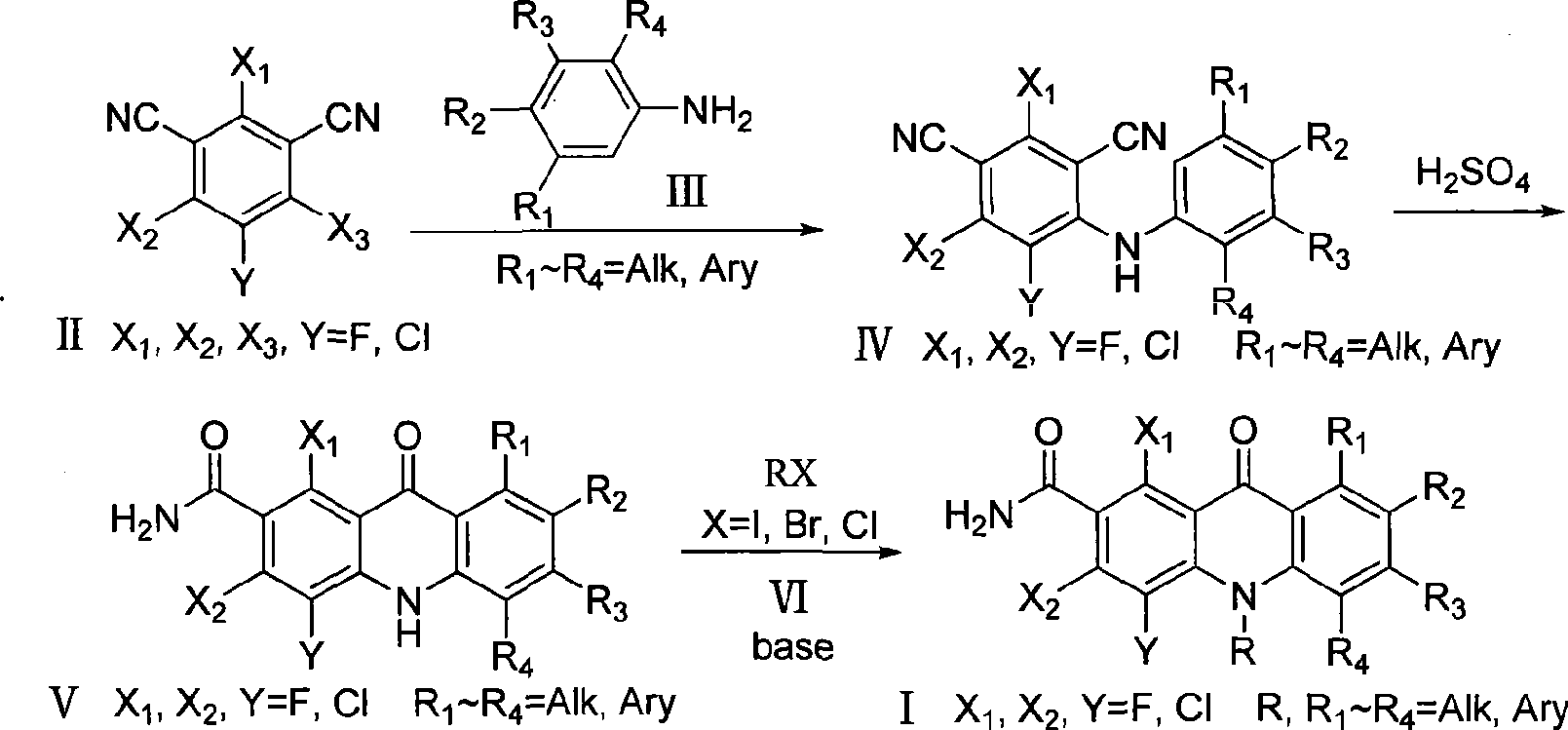
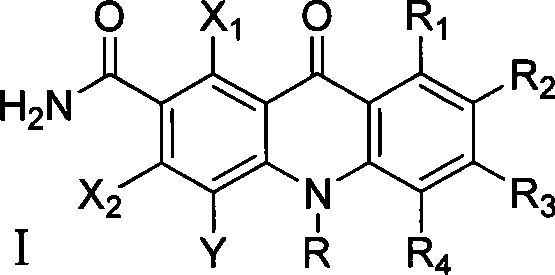
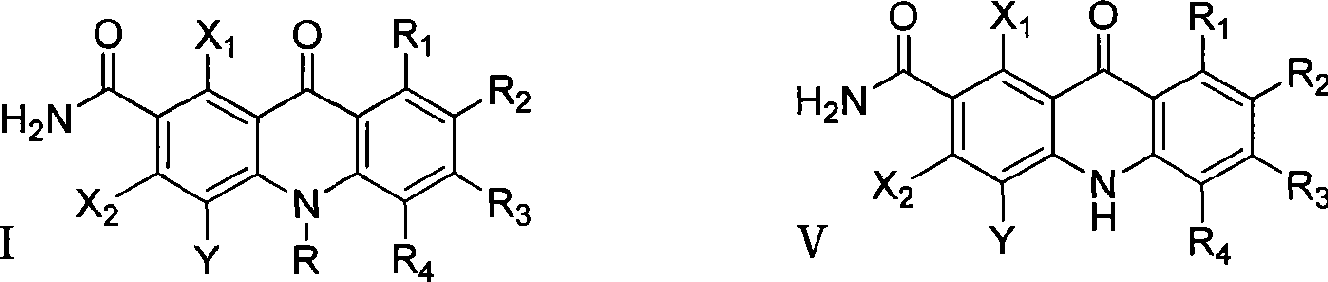Polyhaloacridones compound, intermediate and synthetic method thereof
A polyhalogenated acridone and compound technology, which is applied in the field of polyhalogenated acridone compounds, can solve the problems that the photoelectric properties of acridone derivatives have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] 2,4,5-trichloro-6-phenylamino-1,3-phthalonitrile preparation (IV-A): 10.635 grams (0.04mol) chlorothalonil was added to a 250 ml three-necked bottle, and 150 Milliliter of N, N-dimethylformamide, 7.4 ml of aniline was slowly added under electromagnetic stirring, and the reaction was continued for 8 hours at room temperature. After the reaction was detected by TLC, the reaction solution was poured into 150 ml of water and stirred, and suction filtered to obtain The white solid was dried and recrystallized from acetone to obtain 10.1 g of white solid (yield 78.3%). Melting point: >250°C; MS (m / z, %): 322.0 (M + +H).
Embodiment 2
[0098] Preparation of 5-chloro-2,6-difluoro-6-phenylamino-1,3-phthalonitrile (IV-I): 10.8 grams (0.05mol) of 5-chloro-2,4,6-trifluoro -1,3-phthalonitrile was added to a 250 ml three-necked flask, 150 ml of N,N-dimethylformamide was added, and 10 grams of potassium carbonate was added, and 5 ml of aniline was slowly added under electromagnetic stirring, and at room temperature The reaction was continued for 3 hours. After the reaction was detected by TLC, the reaction solution was poured into 100 ml of water and stirred, and filtered with suction to obtain a white solid. After drying, it was recrystallized with acetone to obtain 13.3 g of a white solid (91.7% yield). Melting point: >250°C; MS (m / z, %): 290.0 (M + +H).
Embodiment 3
[0100] Preparation of 5-chloro-2,6-difluoro-6-p-chloroanilino-1,3-phthalonitrile (IV-J): 10.8 grams (0.05mol) of 5-chloro-2,4,6- Trifluoro-1,3-phthalonitrile was added to a 250 ml three-necked flask, 150 ml of N,N-dimethylformamide was added, 10 grams of potassium carbonate was added, and 4.2 grams of p-chloroaniline was added under electromagnetic stirring. The reaction was continued at room temperature for 18 hours. After the reaction was detected by TLC, the reaction solution was poured into 100 ml of water and stirred, filtered with suction to obtain a white solid. After drying, it was recrystallized with acetone to obtain 13.8 g of a white solid (yield 85.2%). Melting point: >250°C; MS (m / z, %): 324.0 (M + +H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com