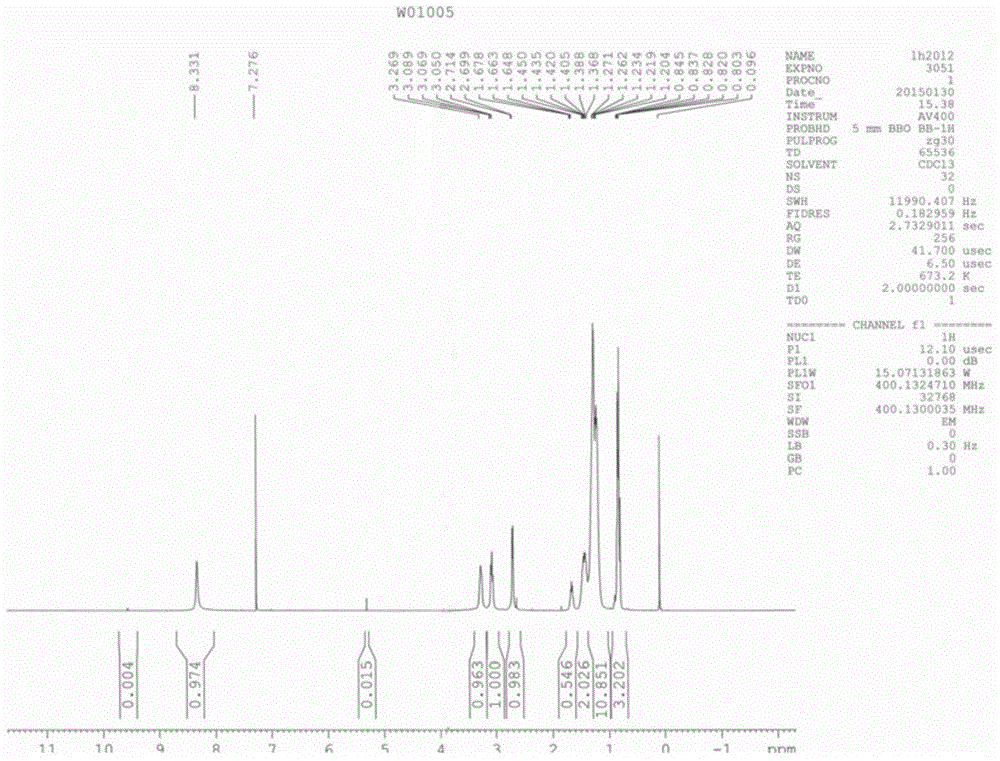
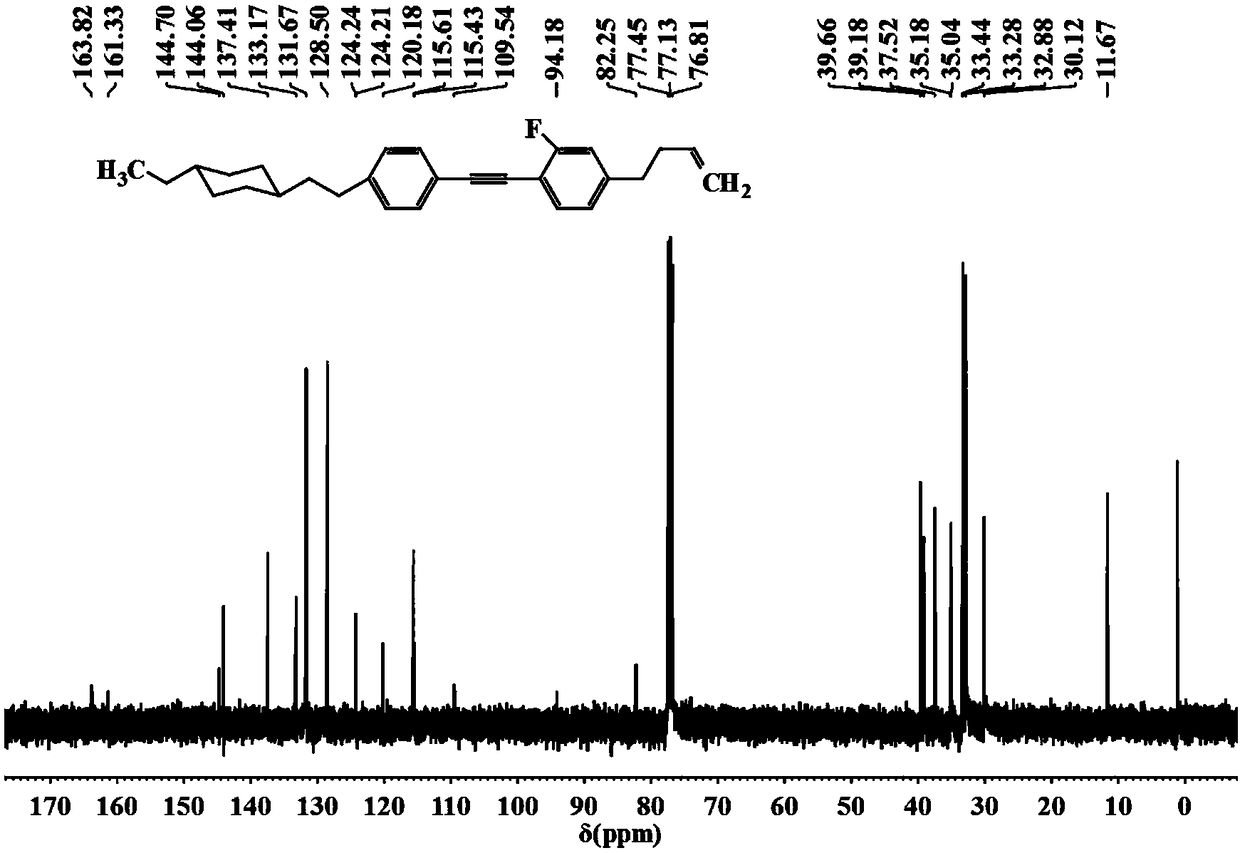
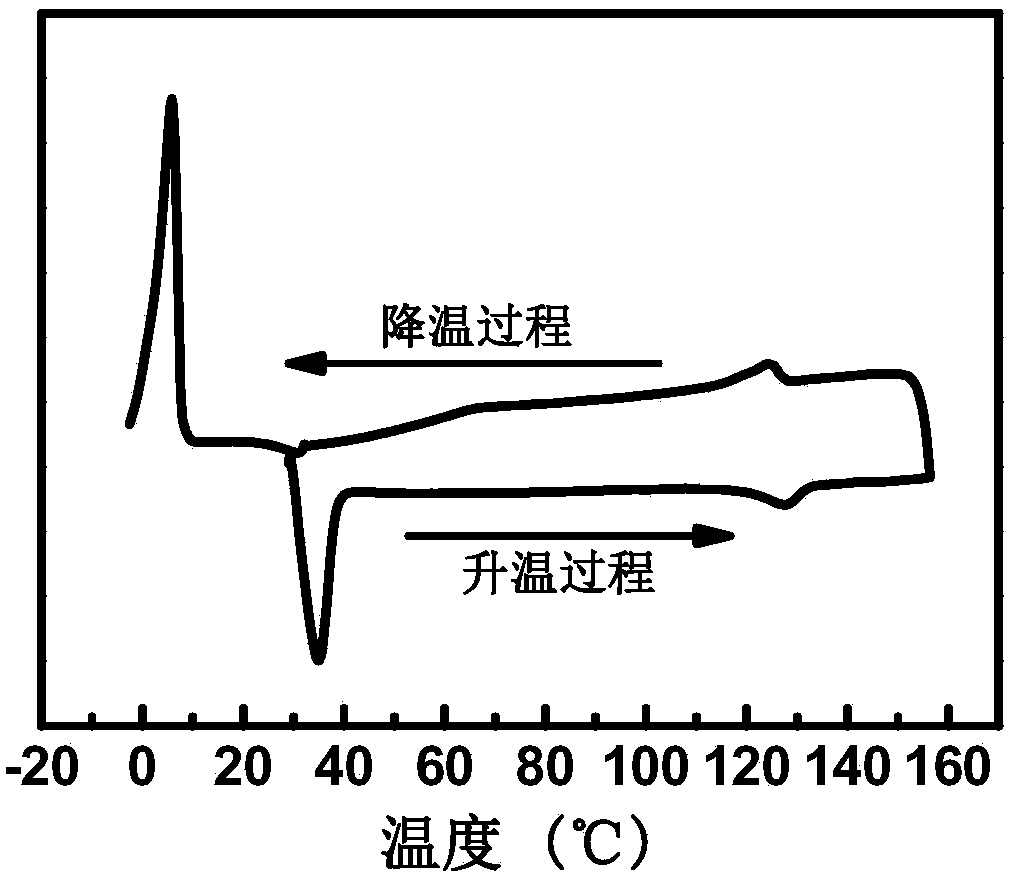
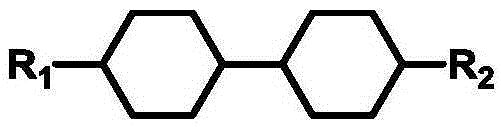
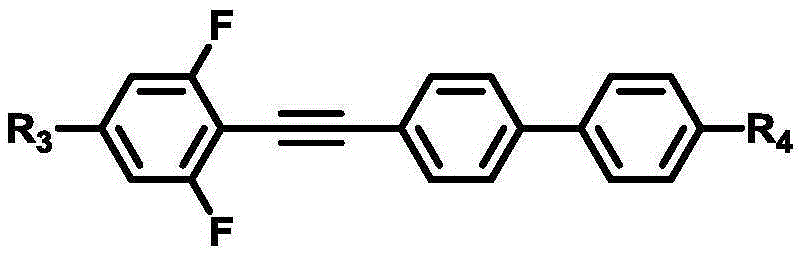
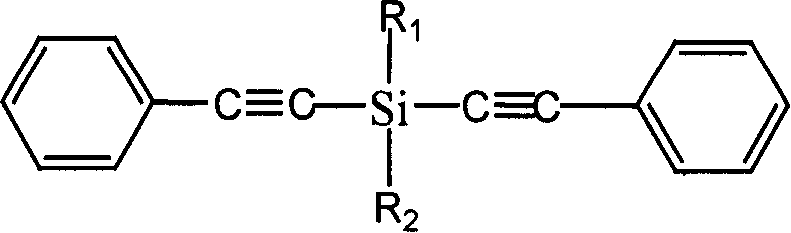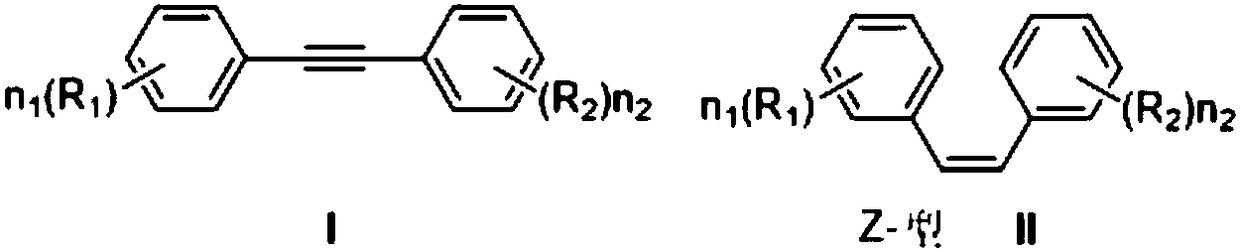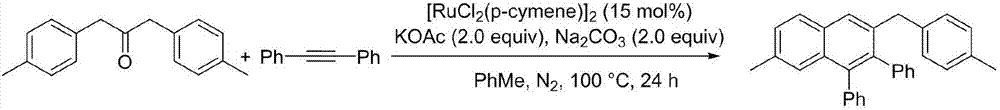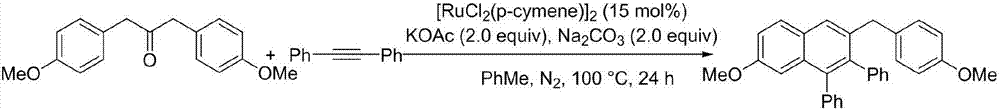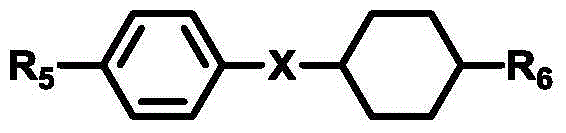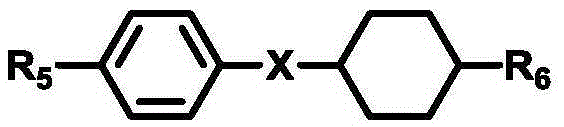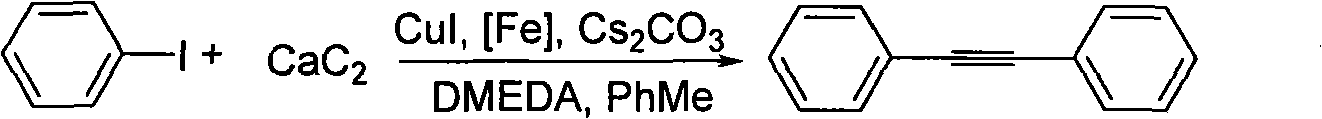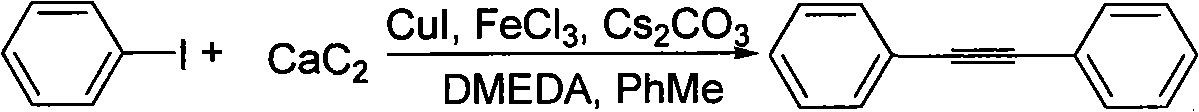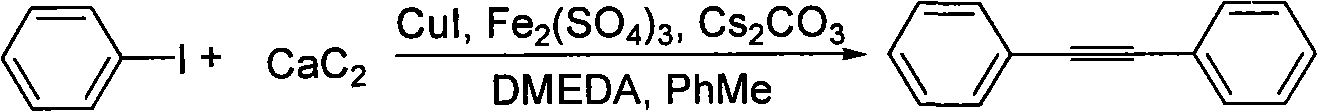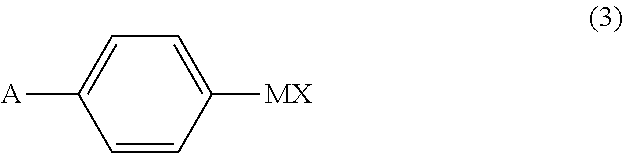Patents
Literature
65 results about "Diphenylacetylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
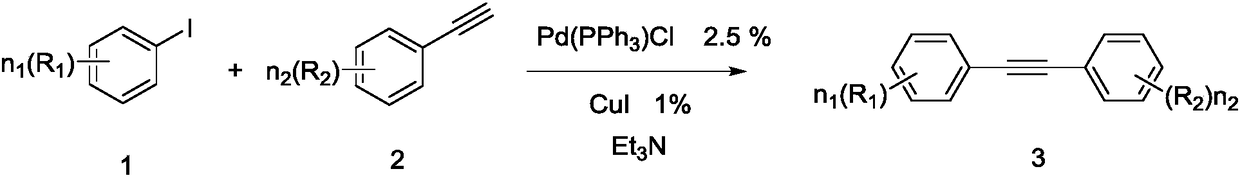
Diphenylacetylene is the chemical compound C₆H₅C≡CC₆H₅. The molecule consists of phenyl groups attached to both ends of an alkyne. It is a colorless crystalline material that is widely used as a building block in organic synthesis and as a ligand in organometallic chemistry.
Diphenylacetylene liquid crystal compounds
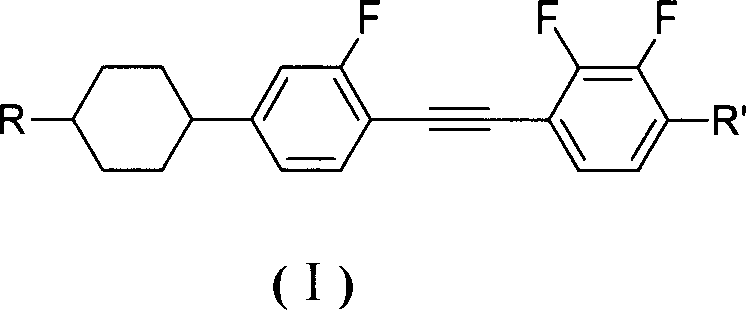
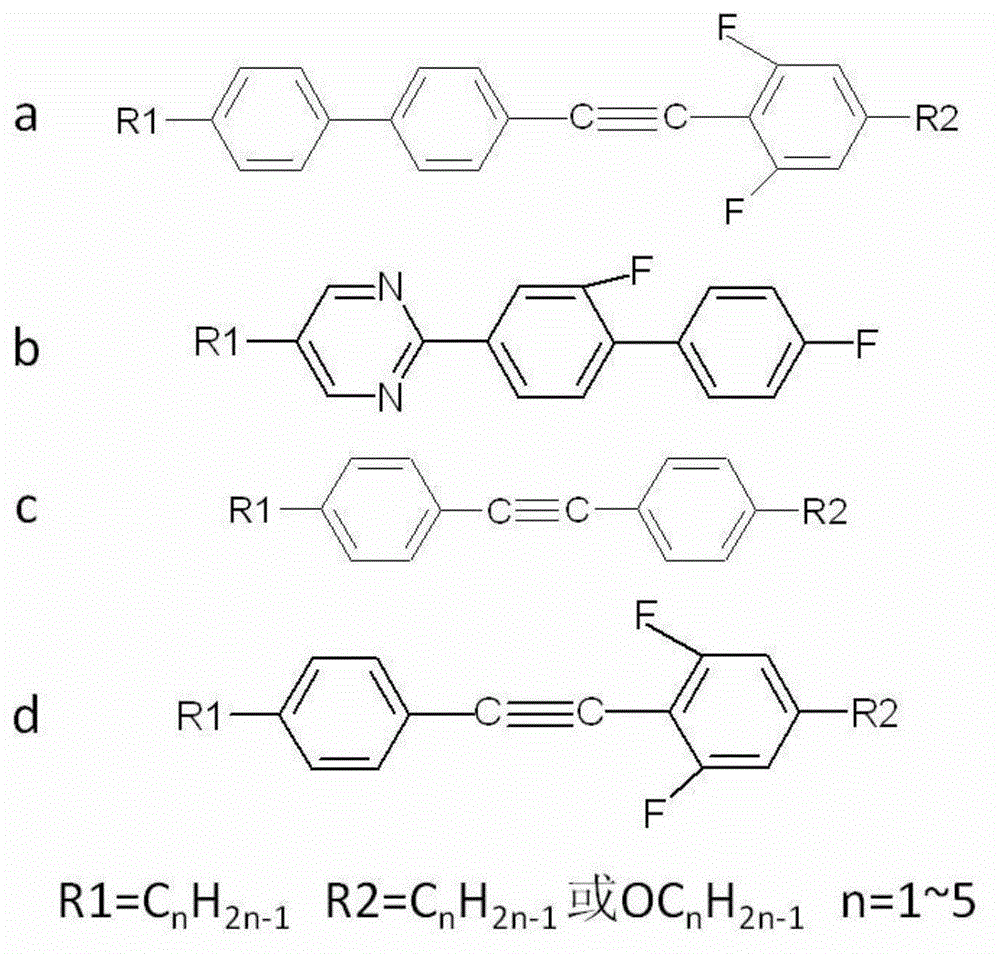
InactiveCN1923951ALarge negative dielectric anisotropySolve the defect of small negative dielectric anisotropyLiquid crystal compositionsNon-linear opticsBenzeneDielectric anisotropy
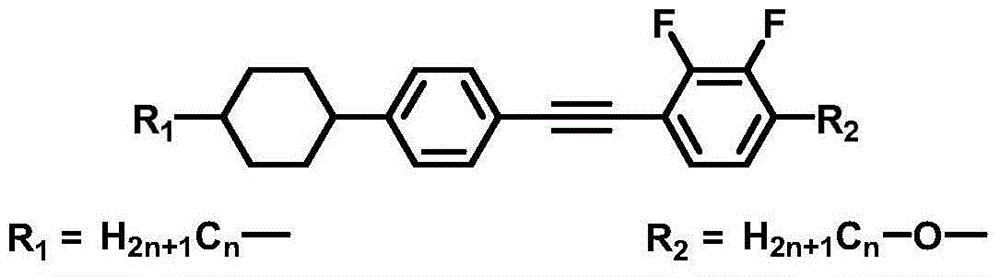
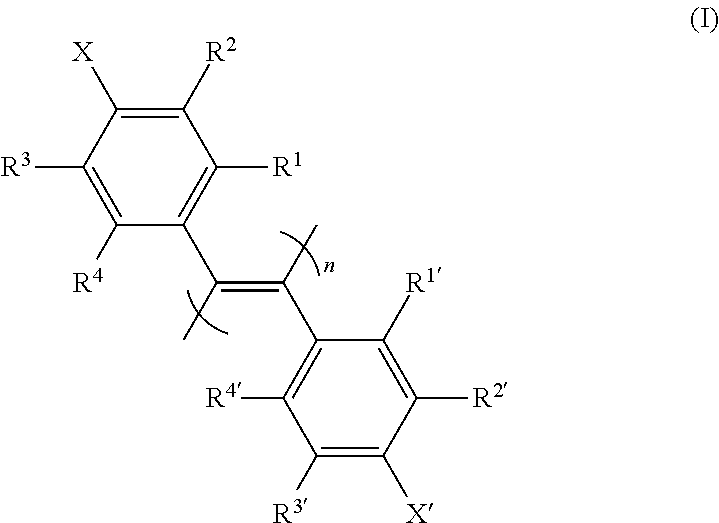
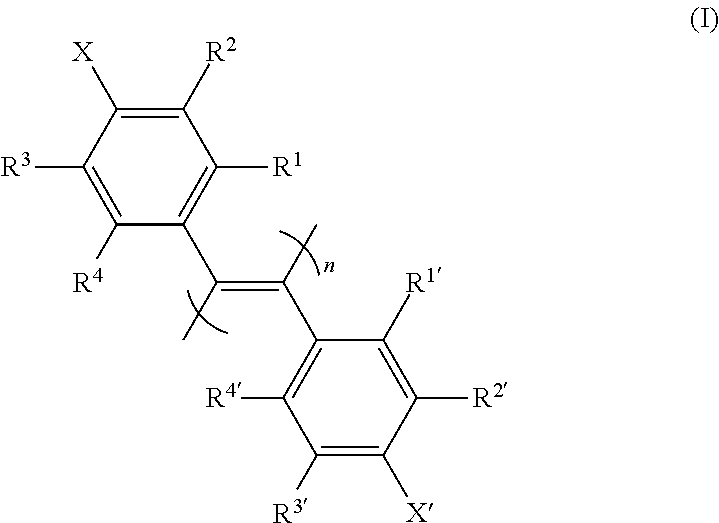
The invention discloses a diphenylacetylene LCD compound to increase negative dielectric aeolotropic value, which possesses structural formula as formula (I), wherein R is C1-9 straight chain normal paraffin; R' is C1-3 straight chain normal paraffin or alkoxy.
Owner:XIAN MODERN CHEM RES INST
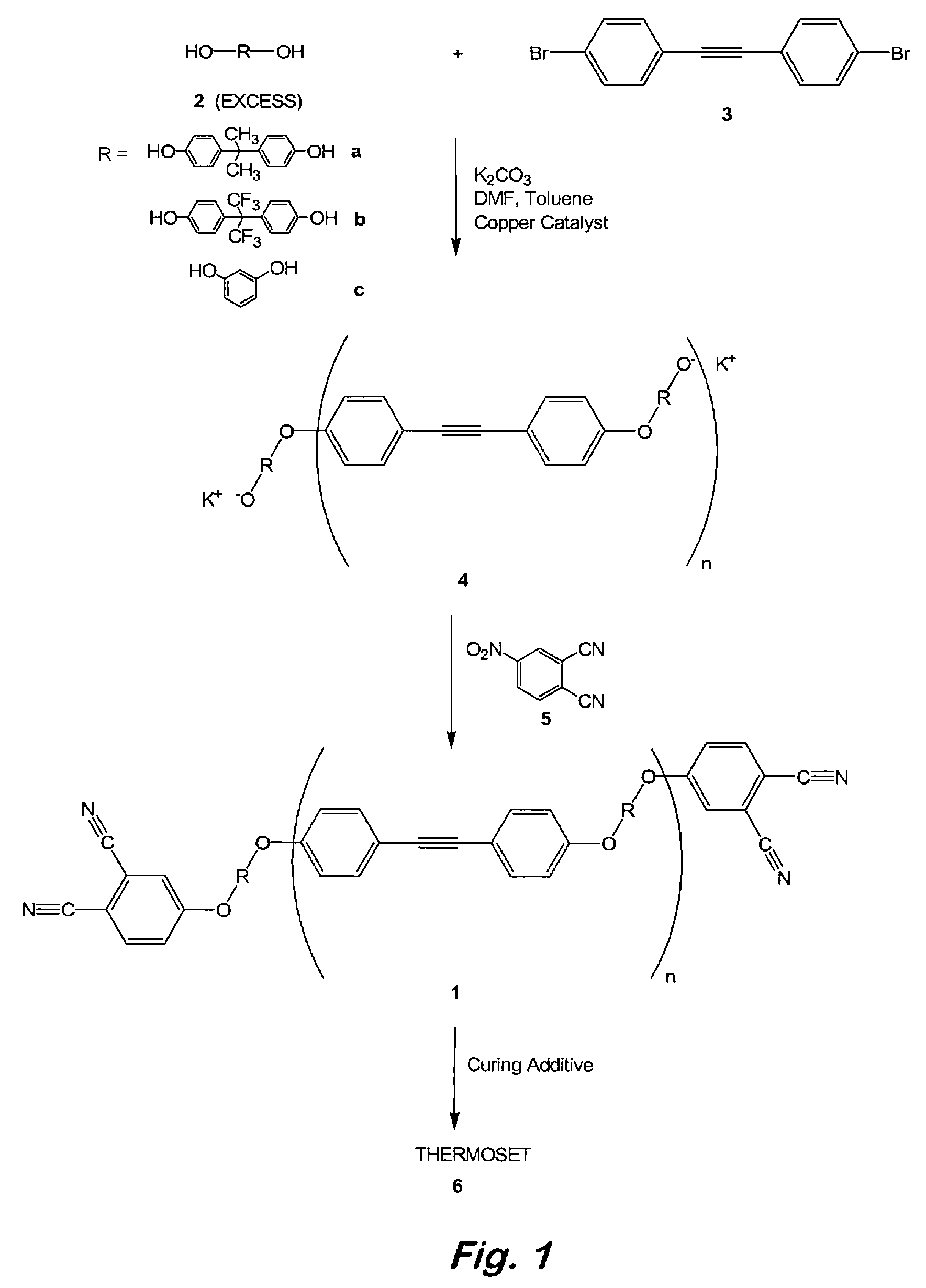
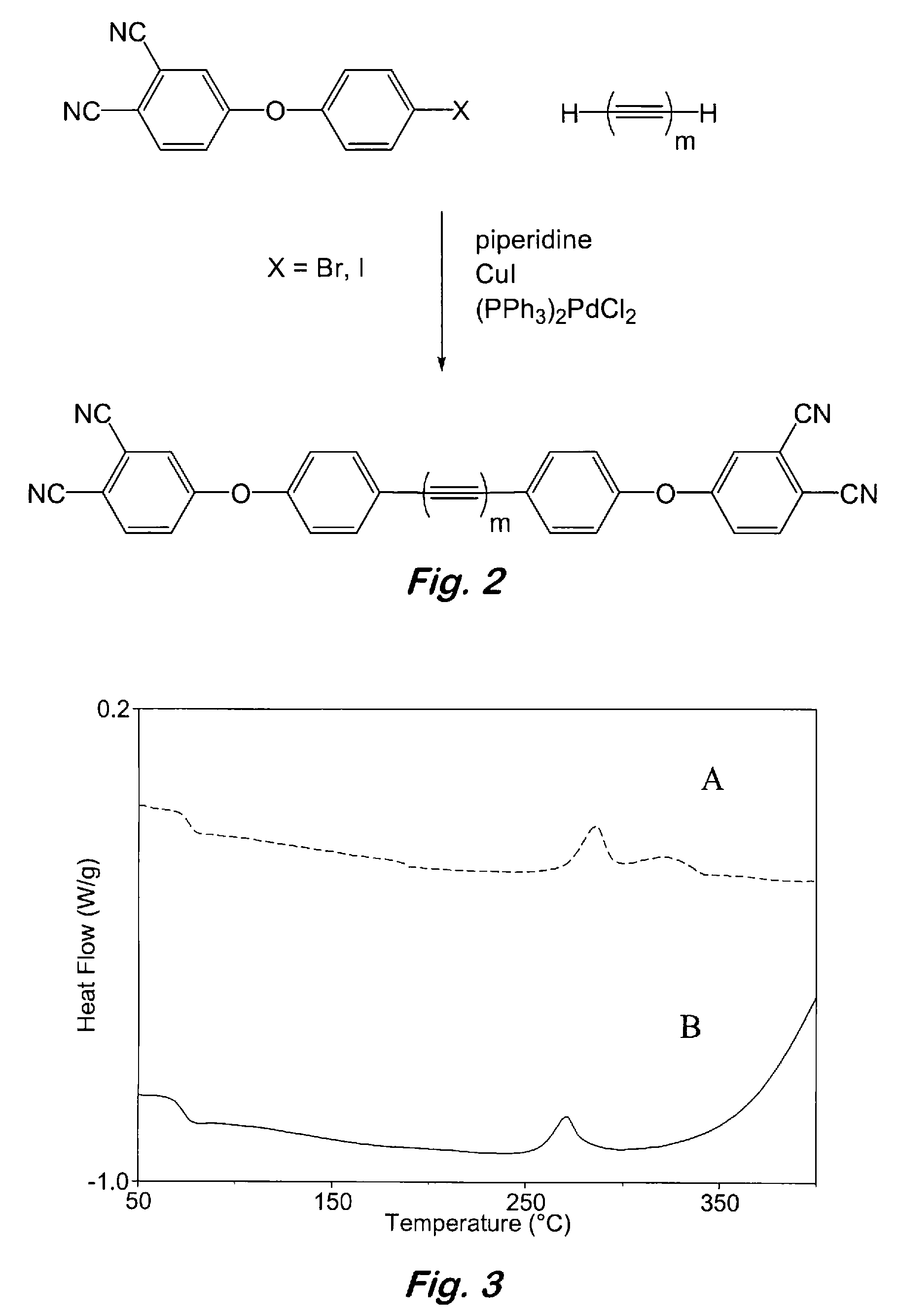
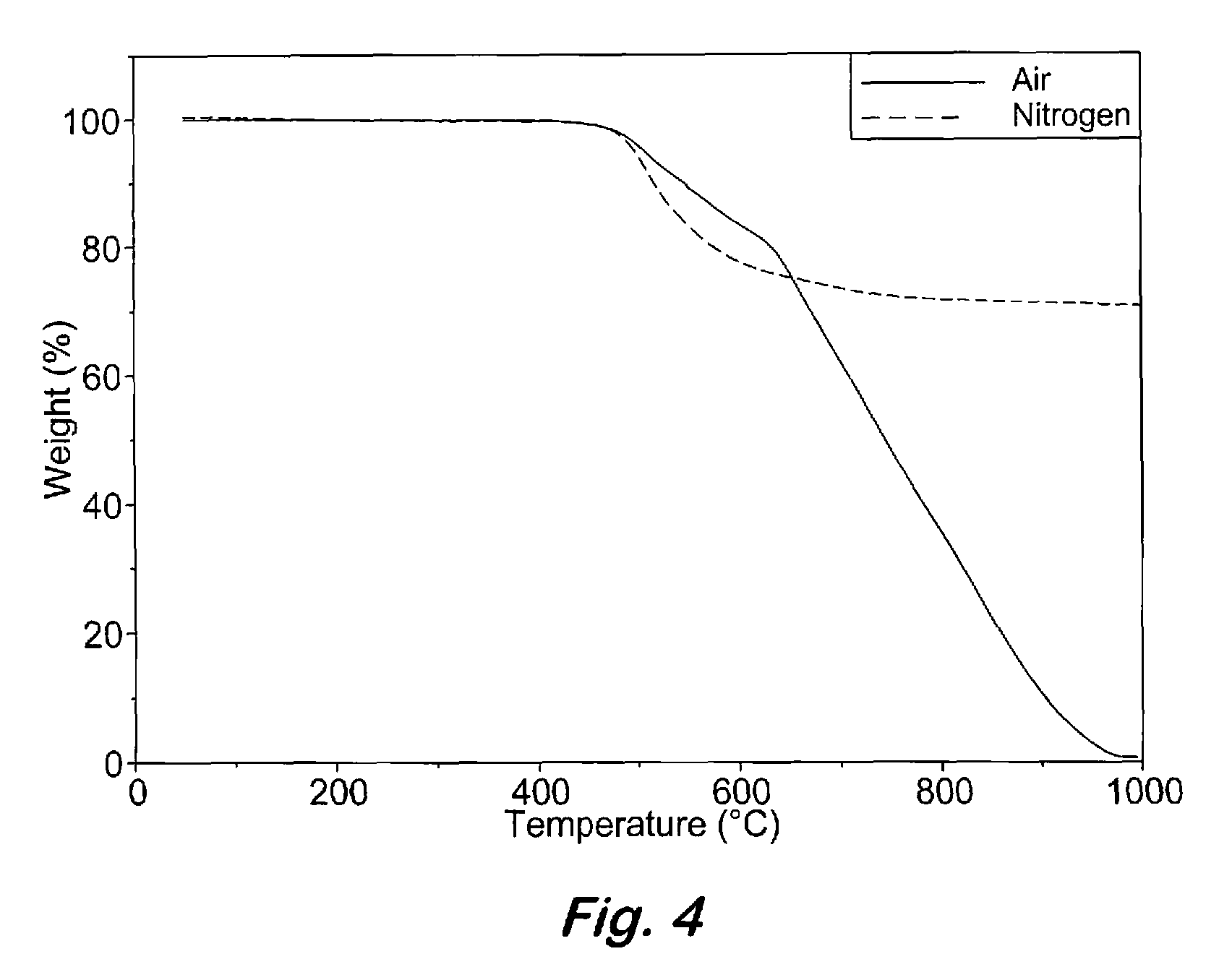
Aromatic ether and alkynyl containing phthalonitriles
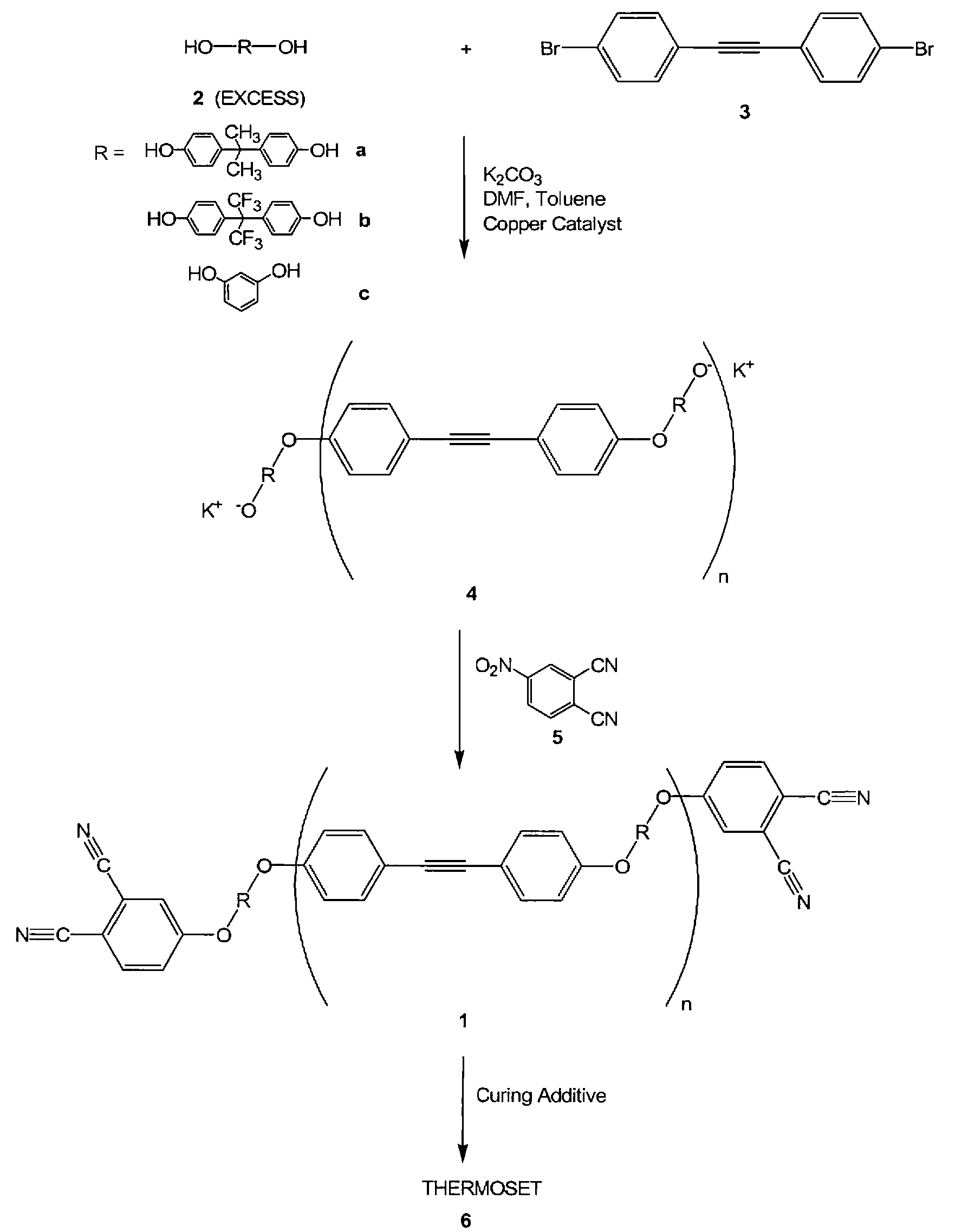
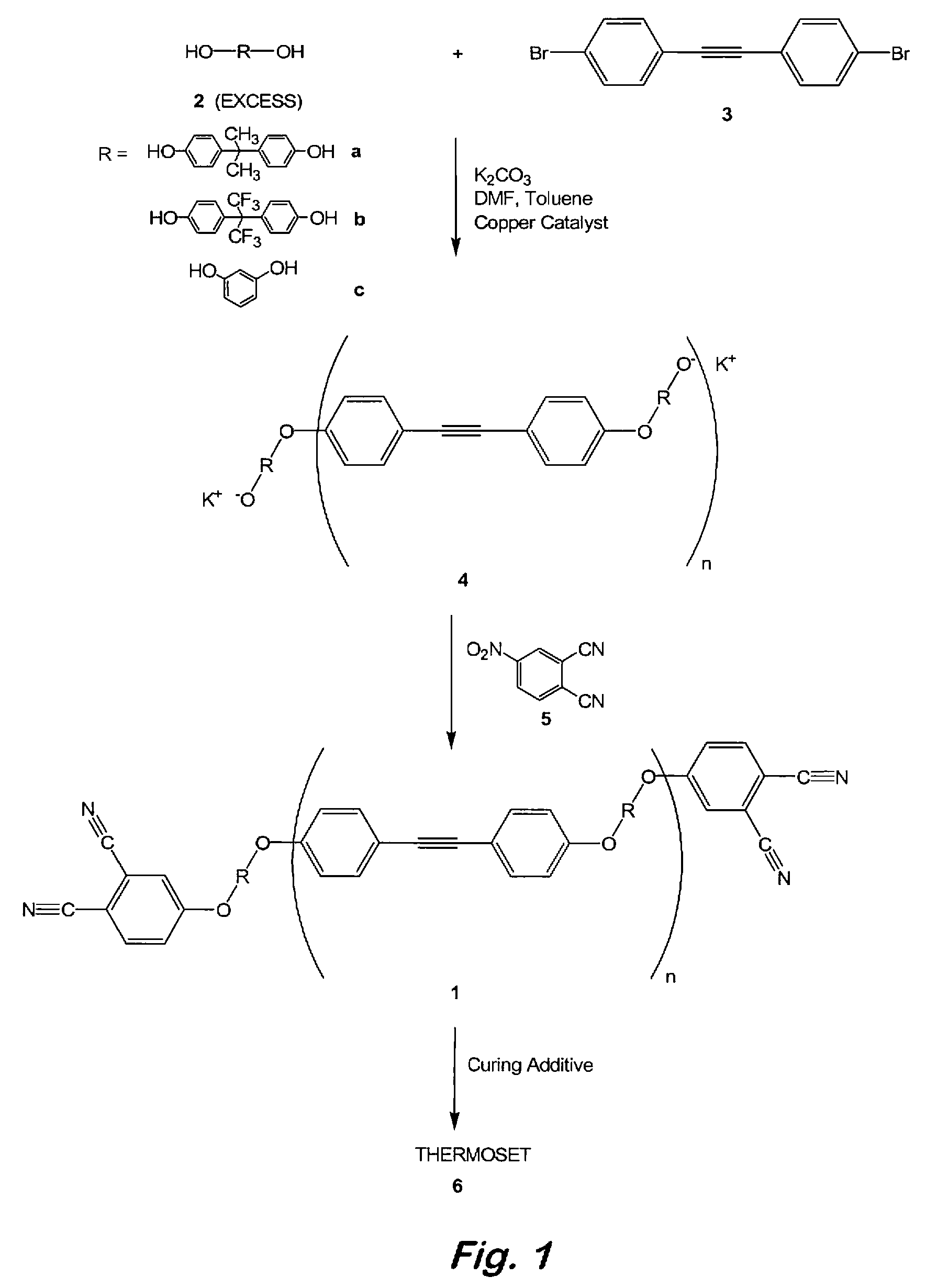
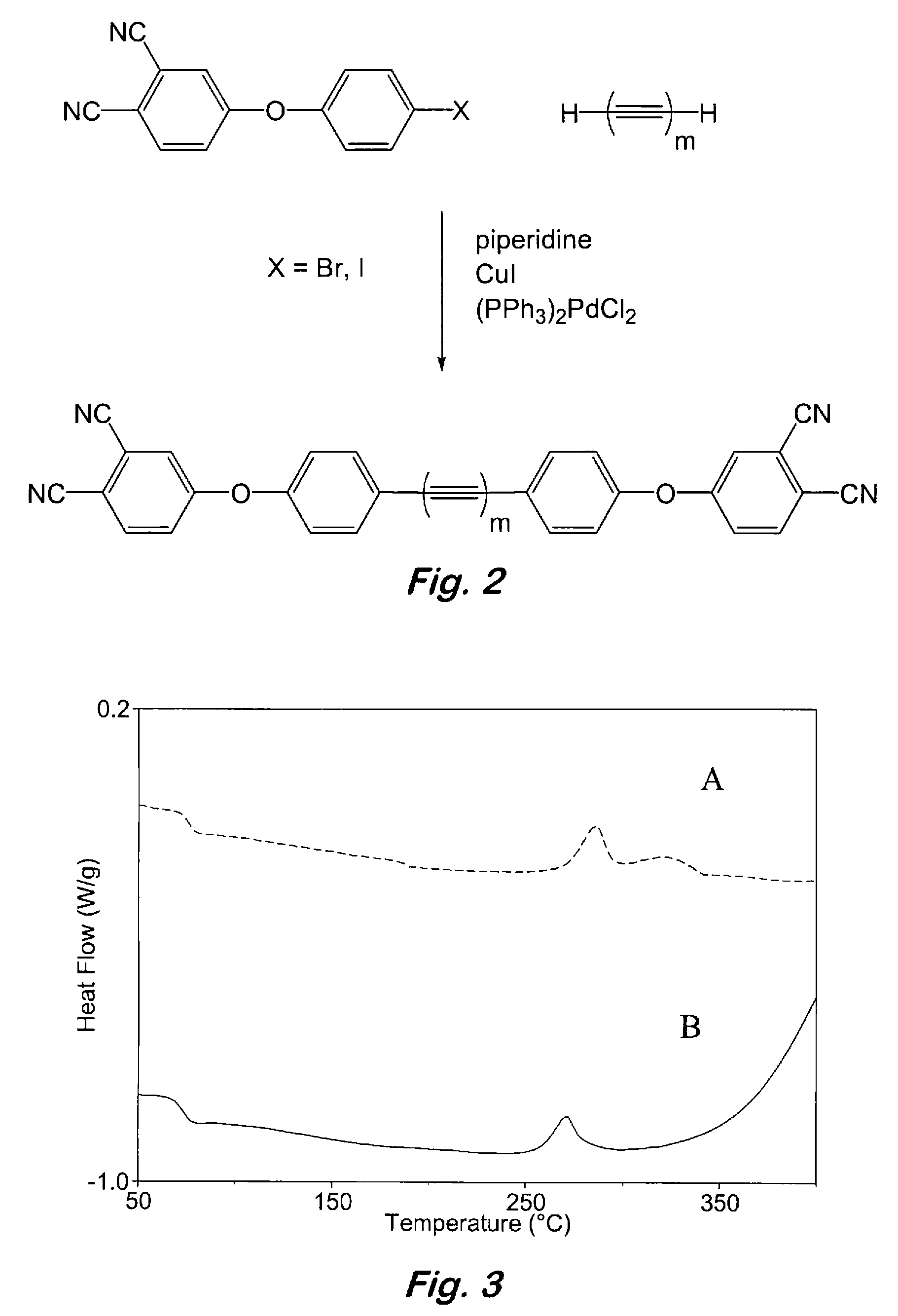
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Polymer dispersed liquid crystal electronic paper and manufacturing method thereof
InactiveCN101329462AAchieve decentralizationEasy to prepareStatic indicating devicesNon-linear opticsComposite filmPolymer science
The invention relates to a polymer dispersed liquid crystal electronic paper and a manufacturing method thereof. A composite film is constituted by two transparent flexible conductive films and a polymer dispersed liquid crystal layer, wherein, electrodes are etched on the two flexible transparent conductive films. A polymer is modified polyurethane acrylate which is added by a surfactant and lecithin, and liquid crystal is dielectric switching liquid crystal material which is the mixture of 20 percent biphenyl ester and 80 percent difluoro diphenylacetylene or the product with the same series. When a low-frequency electric field is imposed on the polymer dispersed liquid crystal electronic paper, the polymer dispersed liquid crystal electronic paper is in the transparent open state, and the transparent open station is still maintained after removing the electric field; when a high-frequency electric field is imposed, the polymer dispersed liquid crystal electronic paper is in the scattering closed state, and the scattering closed state is still maintained after removing the electric field. The manufacturing method of the polymer dispersed liquid crystal electronic paper is to form the weak anchoring polymer which is packaged by a dielectric switching liquid crystal droplet structure by separating after mixing the weak anchoring polymer with the dielectric switching liquid crystal. The manufacturing method combines the natures of the weak anchoring polymer and the dielectric switching liquid crystal with the dual-frequency drive of low-frequency writing and high-frequency erasing so as to realize the stable zero-field of the polymer dispersed liquid crystal, thereby obtaining the new electronic paper technology or being applied as a new product of dimming glass.
Owner:HEBEI UNIV OF TECH
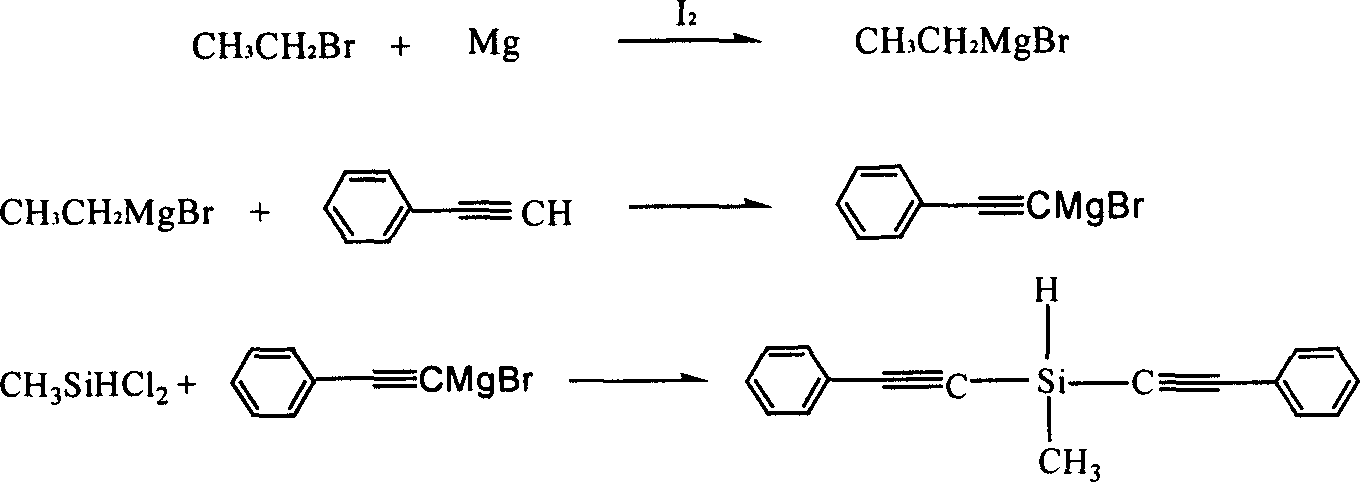
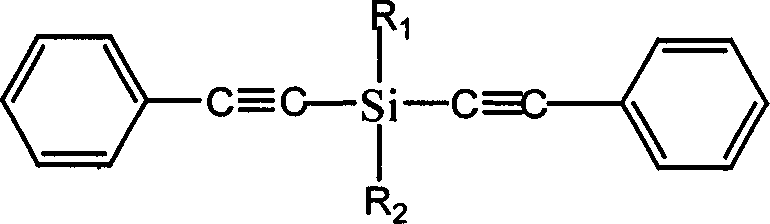
Diphenylacetylene silane novle synthesis method
InactiveCN1763053ASimple processSimple operation processSilicon organic compoundsSilanesSynthesis methods
The present invention is new type of diphenyl acetenyl silane synthesizing process. By using phenyl acetylene, organic lithium reagent and methyl dichlorosilane as material and anhydrous tetrahydrofuran as solvent, the present invention synthesizes methyl diphenyl acetenyl silane monomer through two-step reaction. Phenyl acetylene and butyl lithium first react to produce phenyl acetenyl lithium, and phenyl acetenyl lithium and hydrogen-containing dichlorosilane then react to produce phenyl acetenyl silane. The present invention has simple technological process, simple and feasible operation, short reaction time, controllable reaction condition, high product yield and purity and other advantages, and is suitable for industrial production. The prepared diphenyl acetenyl silane may be used in high performance composite material, ceramic precursor, heat resistant coating, etc.
Owner:EAST CHINA UNIV OF SCI & TECH
Aromatic ether and alkynyl containing phthalonitriles
Compounds having the formulas below. R is an aromatic-containing group. Each M is an alkali metal. Each m is a positive integer. The value of n is a positive integer. The value p is 0 or 1. If p is 0 then n is 1. A thermoset made by curing a composition containing the below phthalonitrile monomers.A method of reacting a diphenyl acetylene compound with an excess of an aromatic diol in the presence of an alkali metal carbonate to form the above oligomer. A method of reacting a phenoxyphthalonitrile with an acetylene compound to form the phthalonitrile monomer below.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
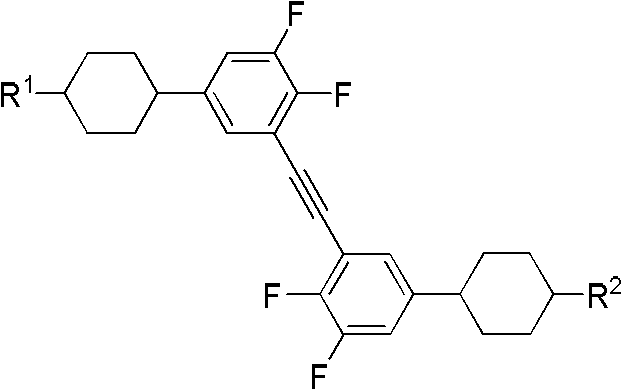
Dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound and preparation method thereof
ActiveCN103805208ALow melting pointLow viscosityLiquid crystal compositionsHydrocarbonsCrystallographyLiquid-crystal display
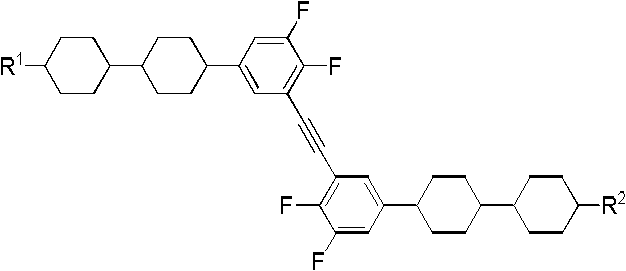
The invention discloses a dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound and a preparation method thereof. The structural general formula of the dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound is shown in the specification, wherein (F)m, (F)n and (F)x respectively represent fluorine atom substitution, m, n and x represent substitution number of the fluorine atoms and the value of m, n and x is 0 or 1, and the cyclohexyl is trans-cyclohexyl; both R and R' represent C1-C15 alkyl, C1-C15 alkenyl, C1-C15 alkoxy, C1-C15 alkenyloxy, fluoro-substituted C1-C15 alkyl or fluoro-substituted C1-C15 alkenyl. The dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound not only has high clearing point, wide nematic phase section, large birefringence, and low optical absorption coefficient, but also has low melting point, low viscosity and good intermiscibility, and can be used in liquid crystal optical elements; the dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound with side fluorine can also be used in a double frequency liquid crystal display mode.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
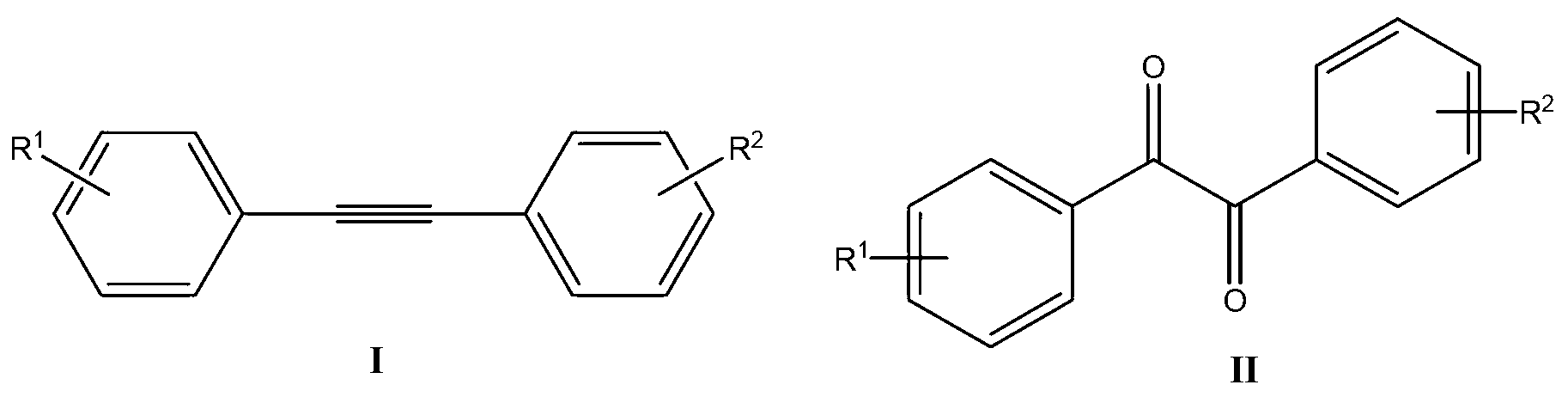
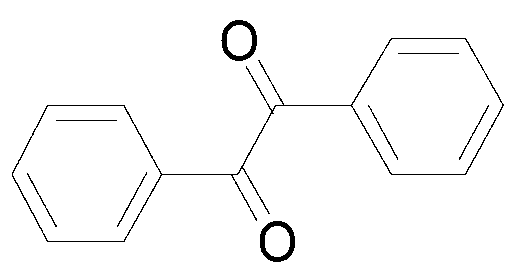
Synthetic method of benzil derivatives
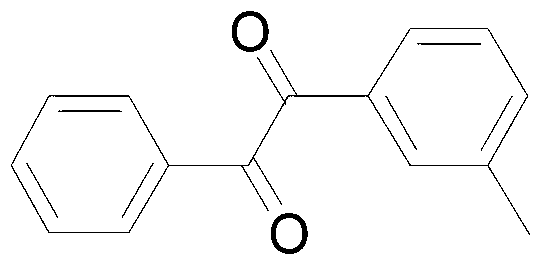
ActiveCN103304393AReduce consumptionLow toxicityOrganic compound preparationCarboxylic acid esters preparationBenzeneDiphenylacetylene
The invention discloses a synthetic method of benzil and derivatives thereof. The diphenylacetylene compound shown in formula I is used as the raw material and is subjected to reaction in a CH3CN / H2O solvent in the presence of a copper catalyst / select flour oxidant system, so as to obtain the benzil derivatives shown in formula II. The synthetic method disclosed by the invention has the advantages of cheap and easily available catalyst, available oxygen source, environment protection, moderate reaction condition, convenient operation and the like, and the functional groups have good universality.
Owner:嘉兴沃特泰科环保科技股份有限公司
Fabrication of carbon nanotube films from alkyne-transition metal complexes
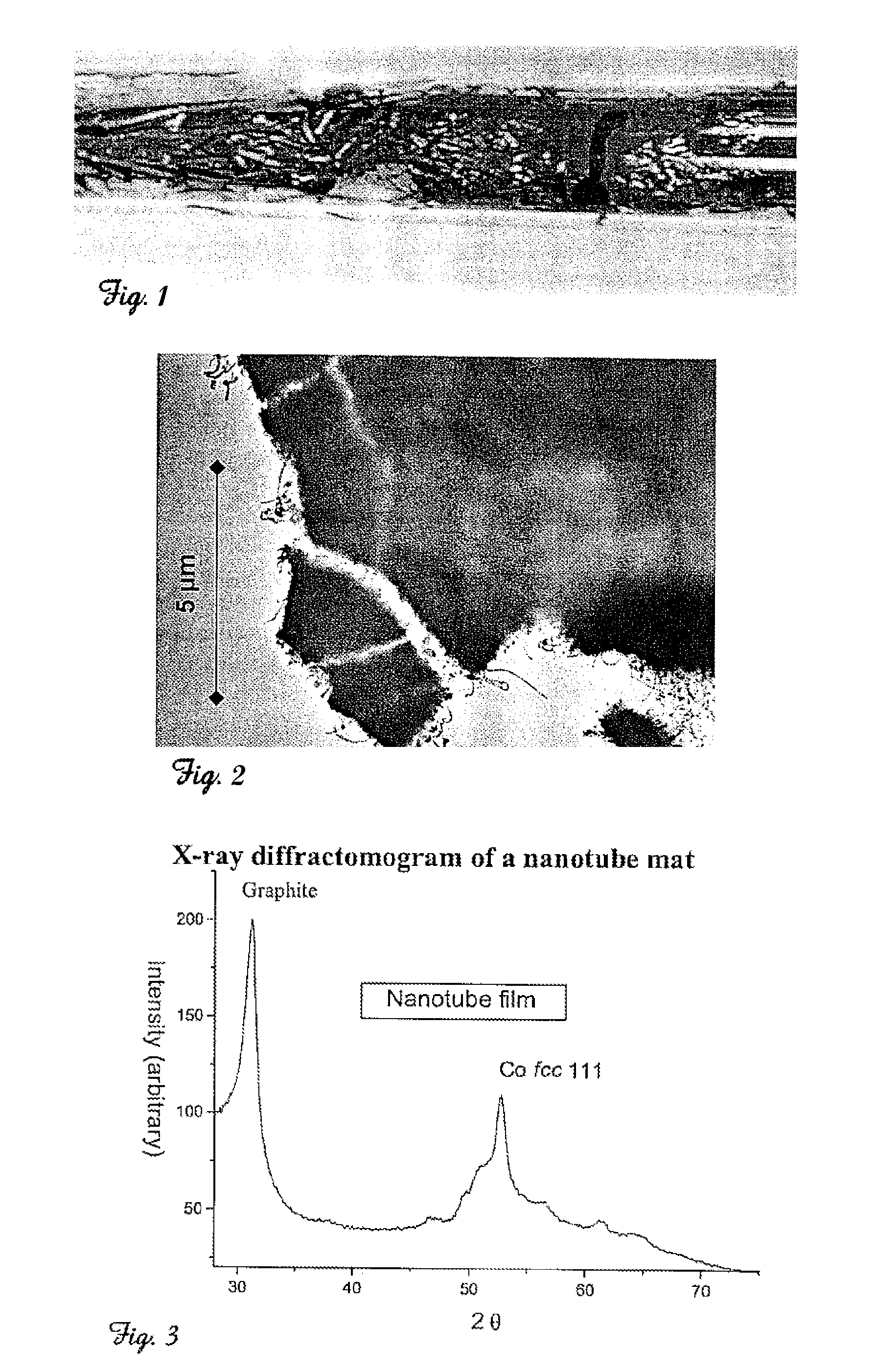
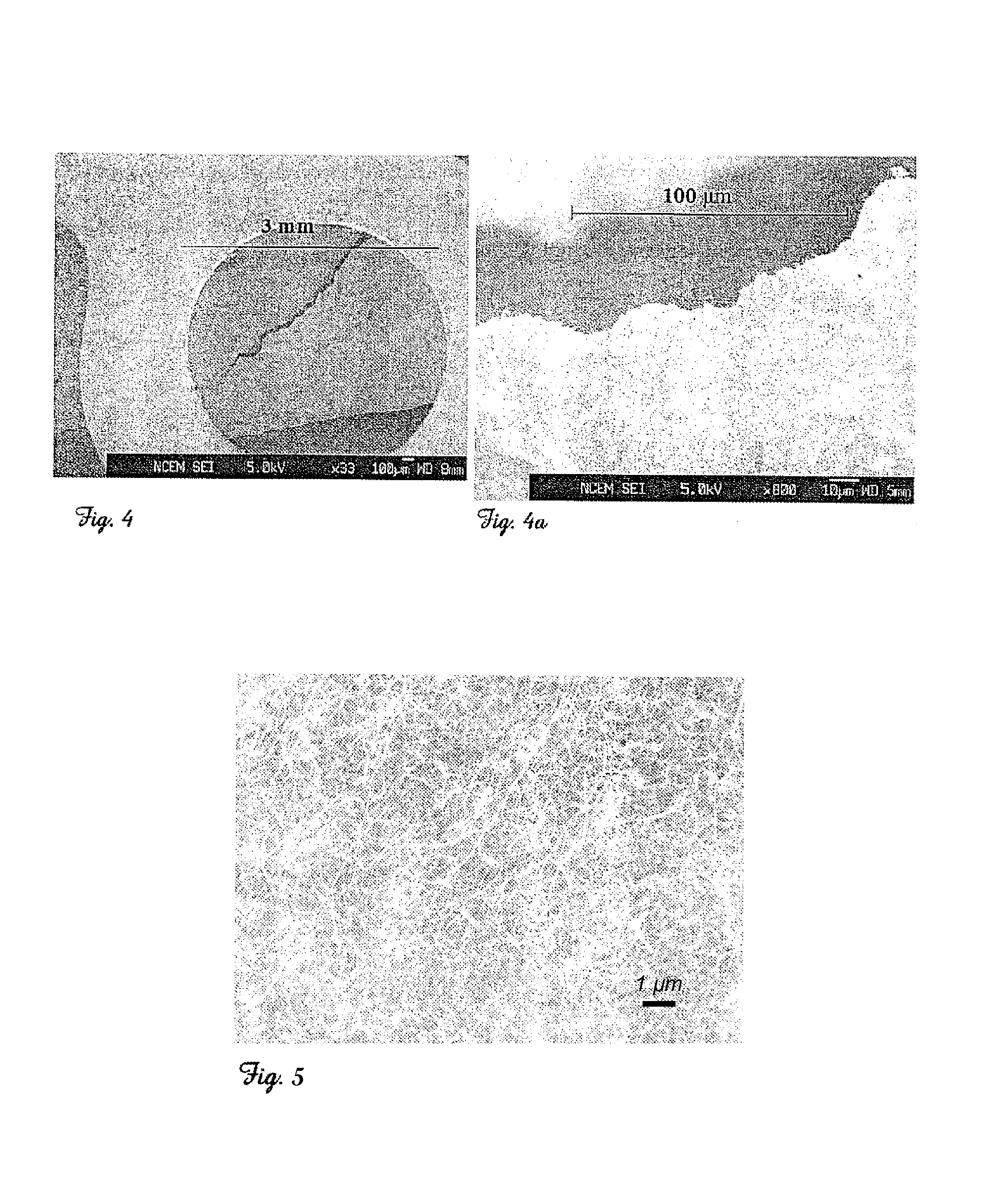
InactiveUS7261871B2Maximize graphitized areaReduce carbon contentMaterial nanotechnologyFibre chemical featuresDiphenylacetyleneAlkyne
A simple method for the production or synthesis of carbon nanotubes as free-standing films or nanotube mats by the thermal decomposition of transition metal complexed alkynes with aryl, alkyl, alkenyl, or alkynyl substituents. In particular, transition metal (e.g. Co, Ni, Fe, Mo) complexes of diarylacetylenes, e.g. diphenylacetylene, and solid mixtures of these complexes with suitable, additional carbon sources are heated in a vessel. More specifically, the heating of the transition metal complex is completed at a temperature between 400-800° C. and more particularly 550-700° C. for between 0.1 to 24 hours and more particularly 0.5-3 hours in a sealed vessel under a partial pressure of argon or helium.
Owner:RGT UNIV OF CALIFORNIA
Method for highly selectively reducing alkyne to generate Z-olefin
ActiveCN109422620AReduce consumptionUniversalCarboxylic acid nitrile preparationOrganic compound preparationDiphenylacetylenePotassium
The invention discloses a method for highly selectively reducing an alkyne to a Z-olefin. The method comprises the steps of adding cuprous chloride, a ligand and potassium t-butoxide to a Schlenk reaction tube, conducting vacuumizing, adding an organic solvent A under protective gas conditions, and conducting uniform stirring at room temperature; and then dissolving a diphenylacetylene compound shown in a formula I and bis(pinacolato)diboron in an organic solvent B to obtain a solution, dropwise adding the solution to the reaction tube, conducting stirring for reaction at room temperature for1-12 hours, and conducting post-treatment on an obtained reaction solution to obtain a Z-olefin and derivatives thereof represented by a formula II, wherein the ligand is 1,3-bis(2,4,6-trimethylphenyl)imidazolium chloride or 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride. The method takes safe cheap ethanol as a hydrogen source, is mild in reaction conditions, saves energy consumption, and inaddition, has the characteristics of high yield, high selectivity, high substrate universality and easy operation.
Owner:ZHEJIANG UNIV OF TECH
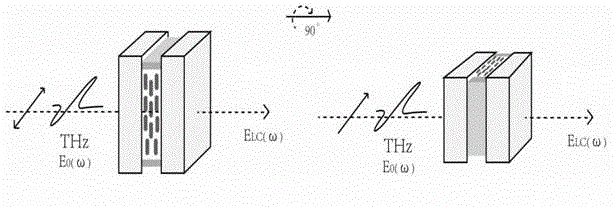
Mixed liquid crystal material having high double-refractivity within terahertz frequency band
ActiveCN102876333AHigh birefringenceLow viscosityLiquid crystal compositionsRefractive indexDiphenylacetylene
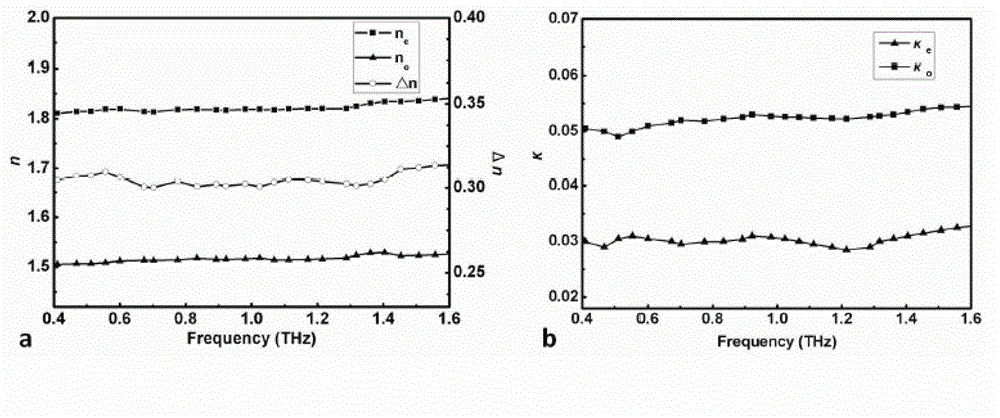
The invention provides a mixed liquid crystal material having high double-refractivity within terahertz frequency band. A series of derivatives R1-PPT (2.6-F)P-R2, accounting for 70-85% and having fluoro-diphenylacetylene skeleton structure, are used as a component a; a series of derivatives R1-P'(3-F)PP-F, accounting for 10-15% and having a fluoro-diphenyl pyridine skeleton structure, are used as a component b; a series of derivatives R1-PTP-R2, accounting for 2-10% and having a diphenylacerylene skeleton structure, are used as a component c; a series of derivatives R1-PT(2,6-F)P-R2, accounting for 2-10% and having a fluoro-diphenylacerylene skeleton structure, are used as a component d; and the components a, b, c and d are subjected to melt blending so as to form a mixed liquid crystal. The the mixed liquid crystal material has the maximum double-refractivity within the terahertz frequency band, has the advantages of wide-temperature liquid crystal phase (from -15 DEG C to 150 DEG C) and low viscosity, can be used for production of quick-response compact THz modulation devices with low working voltage, and is applicable to application fields of material sciences, biomedicines, nondestructive detection and the like in a wide scope.
Owner:NANJING UNIV
Synthetic method of phenytoin sodium
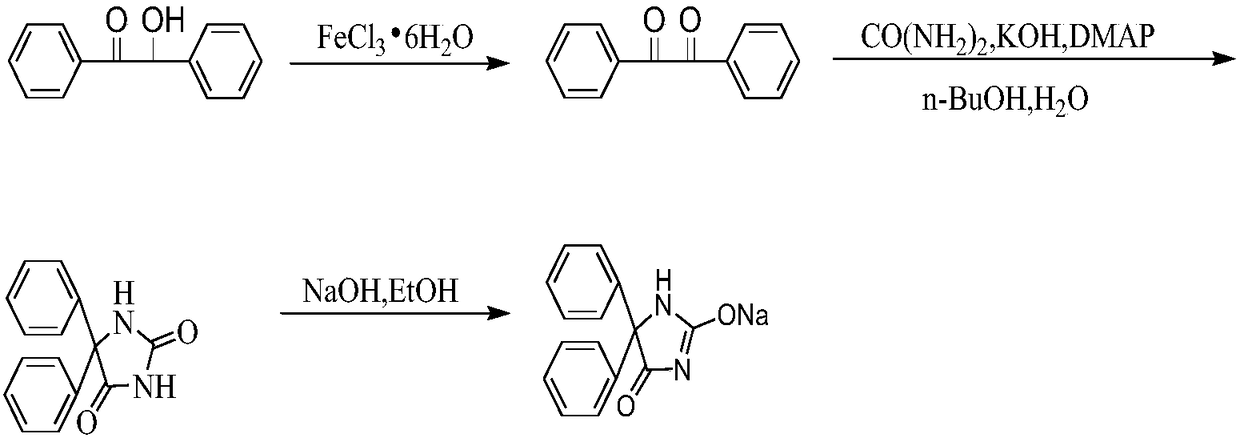
ActiveCN109456271AReduce the reaction concentrationSuppress generationOrganic chemistryDiphenylacetyleneN-Butanol
The invention relates to a synthetic method of phenytoin sodium, belonging to the technical field of biomedicine. The synthetic method of the phenytoin sodium comprises the following steps of oxidation reaction, condensation reaction and salt formation reaction, wherein a amidation reaction process is promoted through adding a phase transfer catalyst 4-dimethylamiopryidine in the condensation reaction; a two-phase system of n-butanol and water is adopted, so that the generation of diphenylacetylene diurea is greatly inhibited, and reaction time is obviously shortened; ethanol serves as a solvent in the salt formation reaction, after a sodium hydroxide ethanol solution reacts with phenytoin, cyclohexane with defective solvent ice is added to promote the phenytoin sodium to precipitate as white crystal, and compared with the salt formation reaction with water as a solvent, the phenytoin sodium is faster in precipitation rate and high in precipitation degree and purity.
Owner:NINGBO POLYTECHNIC
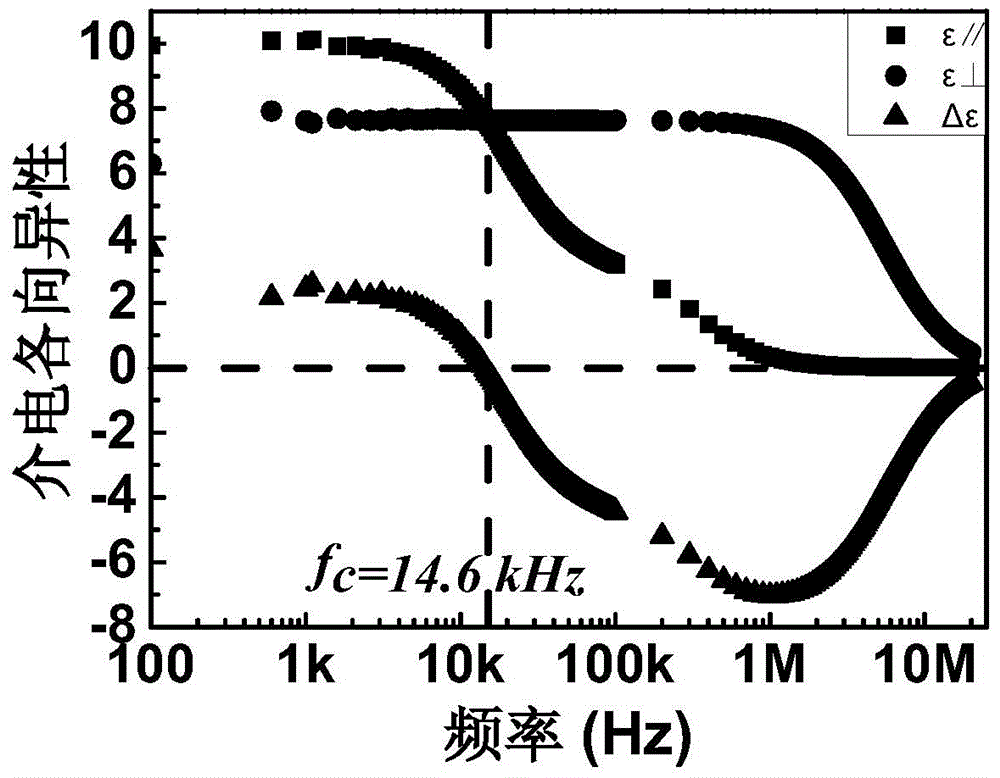
Double-frequency liquid crystal material having low critical frequency
ActiveCN104130782ALow critical frequencyWide liquid crystal phase temperatureLiquid crystal compositionsNon-linear opticsLiquid crystallineBenzene
The invention discloses a double-frequency liquid crystal material having low critical frequency. The double-frequency liquid crystal material comprises 20.0-25.0% of an ingredient A, 20.0-25.0% of an ingredient B, 17.5-22.5% of an ingredient C, 7.5-12.5% of an ingredient D, 7.5-12.5% of an ingredient E, 5.0-10.0% of an ingredient F and 5.0-10.0% of an ingredient G, and the above material are melt and mixed. The ingredient A comprises a series of derivatives having cyclohexylfluorodiphenylacetylene skeleton structures, the ingredient B comprises a series of derivatives having fluorodiphenylacetylene skeleton structures, the ingredient C comprises a series of derivatives having cyclohexylbenzene ring skeleton structures, the ingredient D comprises a series of derivatives having diphenylacetylene skeleton structures, the ingredient E comprises a series of derivatives having three-ring monoester lateral fluoro-group-containing cyano-terminated skeleton structures, and the ingredient F comprises a series of derivatives having three-ring diester lateral dicyano-containing skeleton structures. The double-frequency liquid crystal material has low critical frequency, wide liquid crystalline phase temperature and low viscosity and can be used for preparation of a double-frequency driven liquid crystal device having low work voltage and fast response rate.
Owner:PEKING UNIV
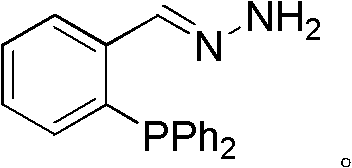
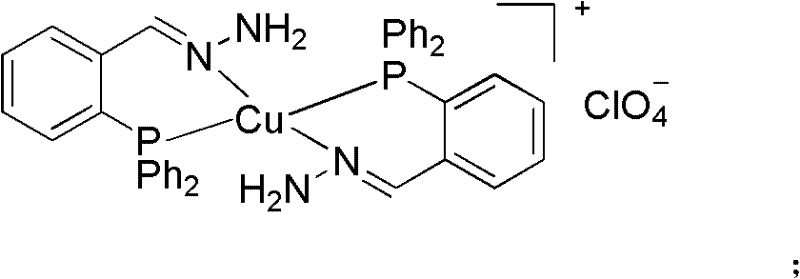
Phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine as well as synthetic method and application of phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine
InactiveCN102603797AHigh reactivityHigh reaction yieldAmino preparation from aminesOrganic compound preparationBenzaldehydeIodide
The invention relates to phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine, which belongs to a diphosphine ligand containing imide groups and contains two atoms coordinated with metal ions. A synthetic method is characterized in that the final target is prepared through preparing an intermediate of 2-diphenylphosphine benzaldehyde; and phosphine imide ligand and cuprous salt form a catalyst system, and the catalyst system is used for cross-coupling catalytic reaction between terminal alkyne and sp2 type carbon halide for preparing substituted diphenylacetylene. The phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine has the advantages that the catalyst system has high reaction reactivity on aryl iodide substrates and also has a certain activity on aryl bromide, the applicable substrate range is wide, and in addition, self-coupling products can be perfectly controlled not to be generated; the synthetic method is simple, and the post treatment is convenient; expensive and toxic palladium is not used in the catalysis, and cheap, economic and clean copper which is easy to obtain is adopted; and organic amine is not used as an alkaline solvent, in addition, the reaction time is short, the catalyst consumption is low, and good application prospects are realized in industrial production.
Owner:NANKAI UNIV +1
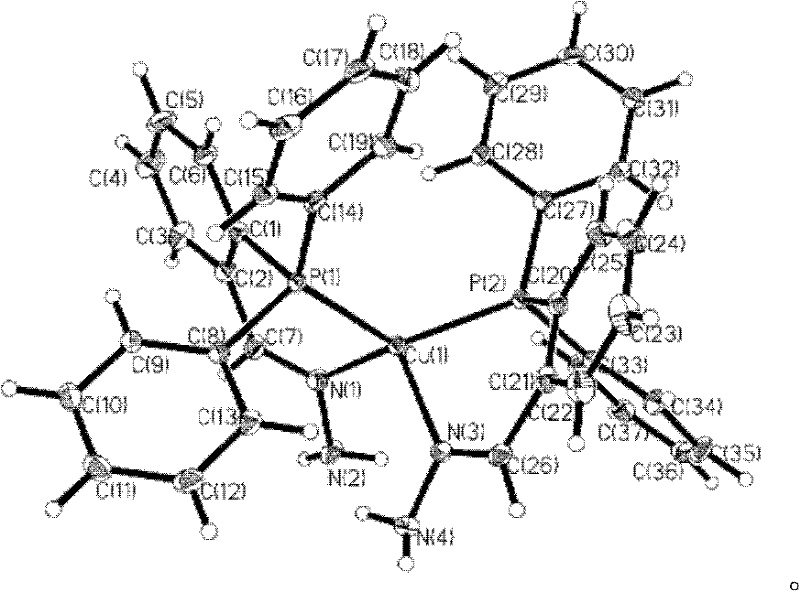
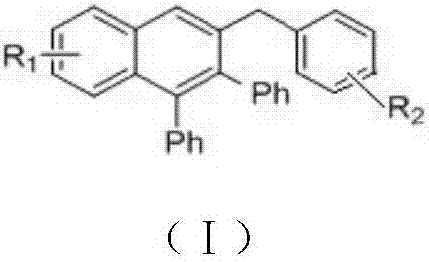
Method for preparing polyaryl substituted naphthalene derivative by ruthenium-catalyzing aromatic ketone and diphenylacetylene cyclization reaction and application
ActiveCN107973691AThe synthesis method is simpleSuitable for large-scale productionOrganic compound preparationHydrocarbonsSynthesis methodsDiphenylacetylene
The invnetion relates to a method for preparing a polyaryl substituted naphthalene derivative by ruthenium-catalyzing aromatic ketone and diphenylacetylene cyclization reaction and application. According to the method provided by the invention, the ruthenium which is relatively cheap is used as a catalyst and aromatic ketone beta-H is activated to synthesize a six-membered ring, i.e., the polyarylsubstituted naphthalene derivative; in a reaction process, an additive and an oxidant do not need to be added and only simple alkali is utilized; the reaction is carried out under moderate reaction conditions. The synthesis method provided by the invention is simple and feasible, scientific and reasonable, green and environmentally friendly and economical and practical and is suitable for being produced in a large scale.
Owner:DALIAN UNIV
Low-threshold low-viscosity nematic phase liquid crystal material as well as preparation method and applications thereof
ActiveCN104152153ALow viscosityLower threshold voltageLiquid crystal compositionsNon-linear opticsDiphenylacetyleneDifluoride
The invention discloses a low-threshold low-viscosity nematic phase liquid crystal material as well as a preparation method and applications thereof. The liquid crystal material is prepared by melt mixing of the following raw materials in percentage by weight 37.57-42.29% of serial derivatives taking dicyclohexyl as a skeleton structure and serving as a component A, 35.13-39.76% of side group difluoro serial derivatives taking diphenylacetylene phenyl as a skeleton structure and serving as a component B, 7.56-12.43% of serial derivatives taking intermediate group-connected cyclohexyl and phenyl as a skeleton structure and serving as a component C, 5.52-9.81% of serial derivatives taking difluoroethylene-connected diphenyl as a skeleton structure and serving as a component D, 0.0-4.38% of serial derivatives taking vinyl difluoride-connected diphenyl as a skeleton structure and serving as a component E, 0.0-4.34% of serial derivatives taking ester group-connected diphenyl as a skeleton structure side group difluoro-terminated cyano and serving as a component F, and 0.0-1.87% of serial derivatives taking cyclohexyl diphenyl as a skeleton structure side group difluoro-terminated fluoro and serving as a component G.
Owner:PEKING UNIV
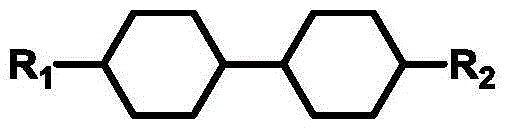
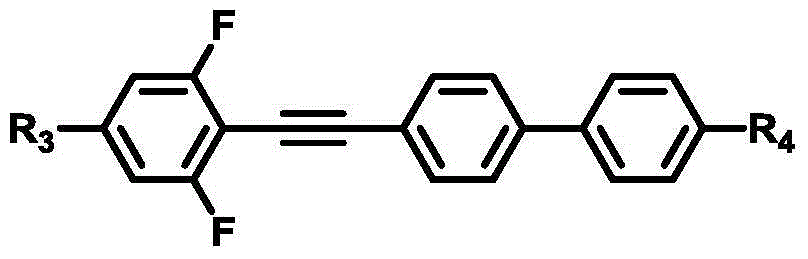
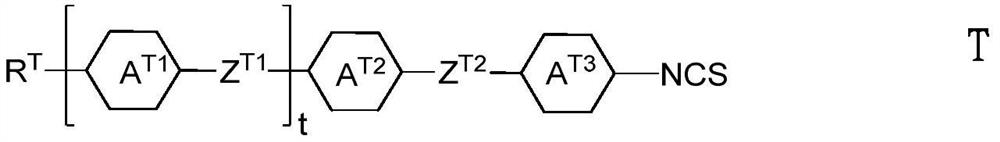
Isothiocyanato-diphenylacetylene
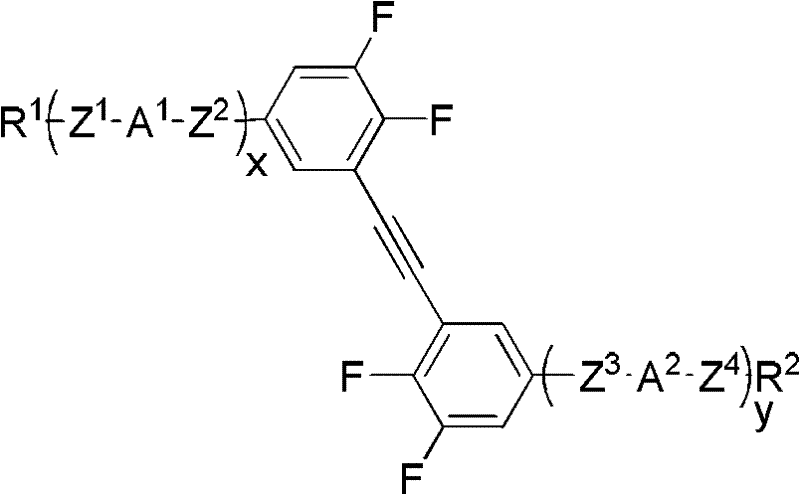
Isothiocyanato-diphenylacetylene. The present invention relates to liquid-crystalline media comprising one or more compounds of formula T as defined in claim 1, and to high-frequency components comprising these media, especially microwave components for high-frequency devices, such as devices for shifting the phase of microwaves, tunable filters, tunable metamaterial structures, and electronic beam steering antennas, e.g. phased array antennas.
Owner:MERCK PATENT GMBH
Hexabenzocoronene compound containing thioether in dovetail-like side-chain and preparation method thereof
ActiveCN104961665AAchieve preparationThe synthesis method is simpleSolid-state devicesSemiconductor/solid-state device manufacturingLiquid crystallineThiourea
The invention relates to a hexabenzocoronene compound containing thioether in the dovetail-like side-chain and a preparation method thereof. The structural general formula of the compound is described in the specification, and in the formula, R is a dovetail-like alkyl group. According to the invention, a ring trimerization-ferric trichloride co-coronene process is employed, 2-(4'-bromophenyl)ethanol is used as a raw material; mercaptan is produced after hydroxyl bromination and thiourea salt reaction; then thioether is prepared; a palladium catalyzed direct coupling reaction is selected so as to obtain a diphenylacetylene derivative; the diphenylacetylene derivative undergoes ring trimerization under the catalysis of carbonyl cobalt so as to produce corresponding hexaphenylbenzene; and hexaphenylbenzene is oxidized with ferric chloride anhydrous in dichloromethane and nitromethane and then reduced with a system consisting of iodine and sodium borohydride, then methanol is added, precipitation is carried out so as to obtain a yellow solid, and the yellow solid is purified. The dovetail-like side-chain is favorable for reduction of the melting point of the compound and enables a compound with a certain chain length to have the properties of discotic liquid crystals and to have mesogenic behavior, so reference is provided for designing of discotic liquid crystals. The hexabenzocoronene compound is extensively applied to organic light-emitting diodes, field effect transistors and solar cells.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
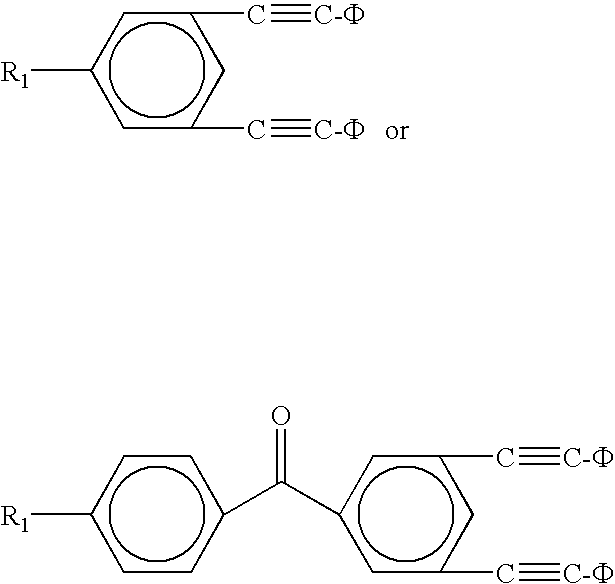
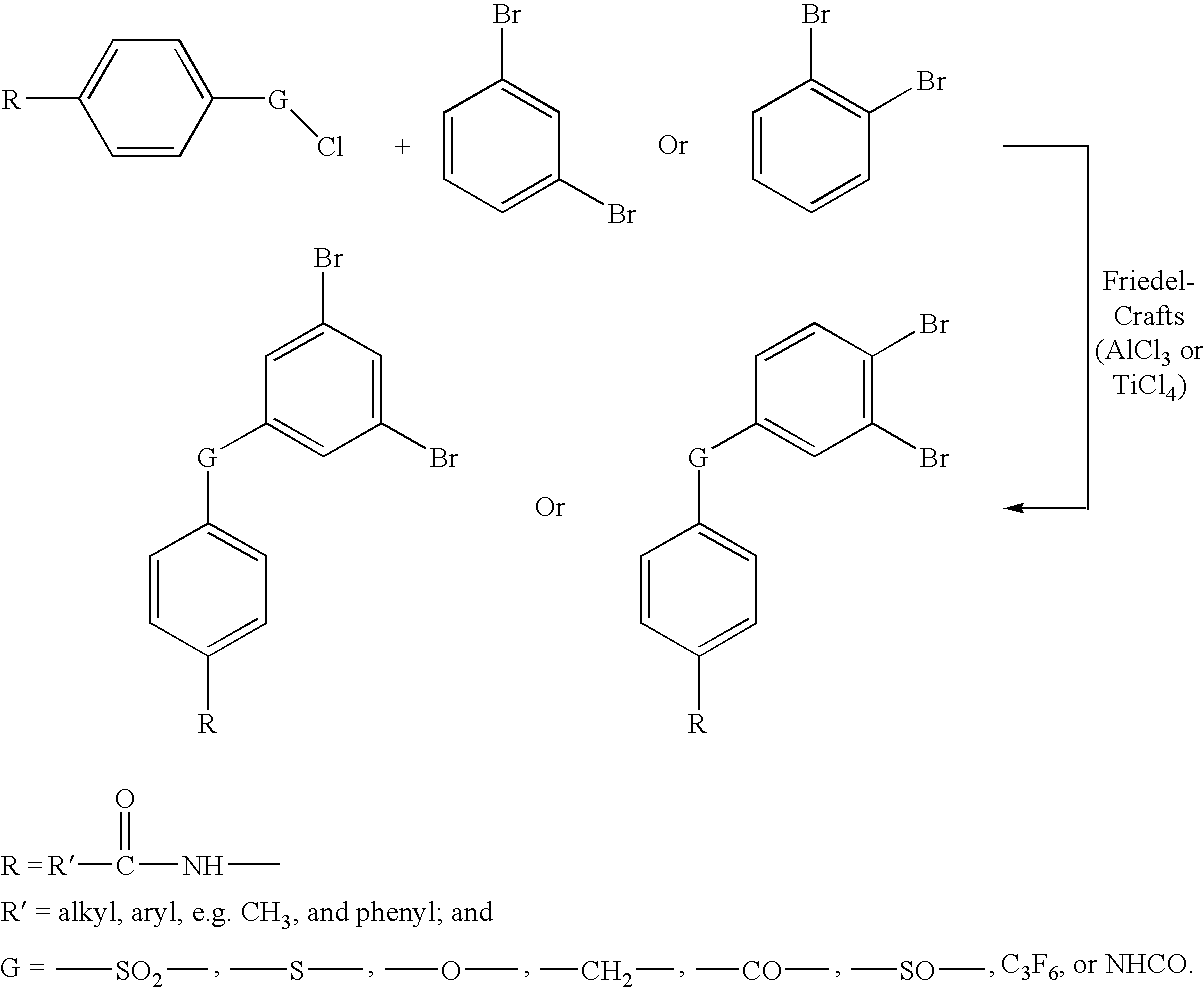
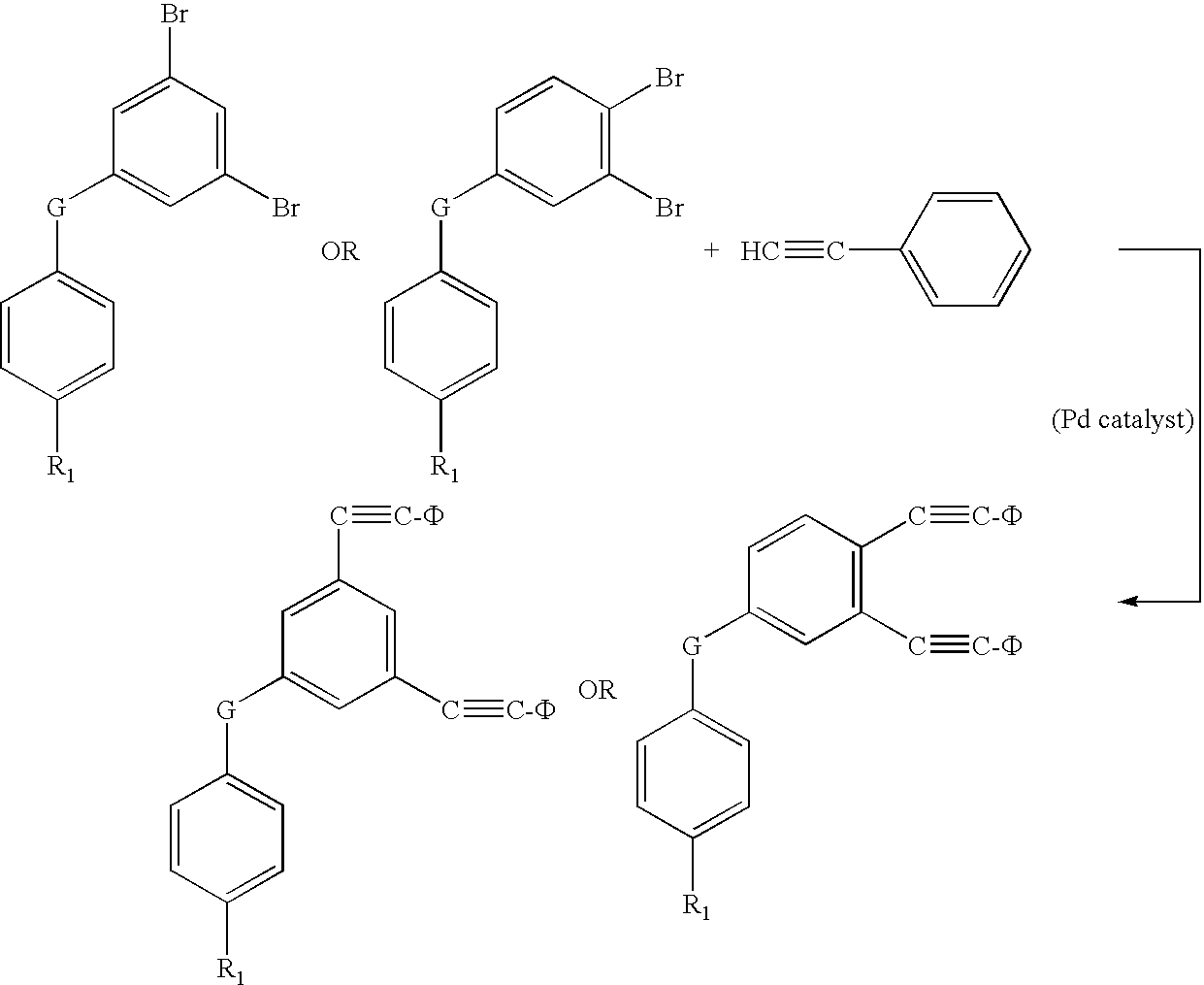
Oligomers with di-phenylethynyl endcaps
An oligomer having di-phenylethynyl endcaps is disclosed. The capped oligomer has the formula:D-A-DwhereinD is a di-phenylethynyl endcap; andA is an oligomer selected from the group consisting of imidesulfone; ether; ethersulfone; amide; imide; ester; estersulfone; etherimide; amideimide; oxazole; oxazole sulfone; thiazole; thiazole sulfone; imidazole; and imidazole sulfone.
Owner:THE BOEING CO
Oligomers with di-phenylethynyl endcaps
An oligomer having di-phenylethynyl endcaps is disclosed. The capped oligomer has the formula:D-A-DwhereinD is a di-phenylethynyl endcap; andA is an oligomer selected from the group consisting of imidesulfone; ether; ethersulfone; amide; imide; ester; estersulfone; etherimide; amideimide; oxazole; oxazole sulfone; thiazole; thiazole sulfone; imidazole; and imidazole sulfone.
Owner:THE BOEING CO
Diphenylacetylene butene liquid crystal compound and synthesis method thereof
InactiveCN108165278ALow melting pointBroaden the nematic rangeLiquid crystal compositionsButeneCrystallography
The invention discloses a diphenylacetylene butene liquid crystal compound and a synthesis method thereof. The structural formula of the compound is shown as the specification, wherein (F)x and (F)y both represent fluorine atom substitution, x and y indicate the substitution number of fluorine atom, and can be 0 or 1, and R represents C1-C15 alkyl or C1-C15 alkoxy. The method provided by the invention employs acetal deprotection and Witting reaction to prepare the diphenylacetylene butene liquid crystal compound, the preparation method is simple in operation, the product yield and purity are high, and the obtained compound has the characteristics of low melting point, low viscosity, wide liquid crystal phase interval, good low temperature performance, direct preparation of liquid crystal mixture and the like, and is suitable for liquid crystal display, especially for TFT liquid crystal display technology.
Owner:SHAANXI NORMAL UNIV
A low-threshold low-viscosity nematic liquid crystal material and its preparation method and application
ActiveCN104152153BLow viscosityLower threshold voltageLiquid crystal compositionsNon-linear opticsDiphenylacetyleneDifluoride
The invention discloses a low-threshold low-viscosity nematic phase liquid crystal material as well as a preparation method and applications thereof. The liquid crystal material is prepared by melt mixing of the following raw materials in percentage by weight 37.57-42.29% of serial derivatives taking dicyclohexyl as a skeleton structure and serving as a component A, 35.13-39.76% of side group difluoro serial derivatives taking diphenylacetylene phenyl as a skeleton structure and serving as a component B, 7.56-12.43% of serial derivatives taking intermediate group-connected cyclohexyl and phenyl as a skeleton structure and serving as a component C, 5.52-9.81% of serial derivatives taking difluoroethylene-connected diphenyl as a skeleton structure and serving as a component D, 0.0-4.38% of serial derivatives taking vinyl difluoride-connected diphenyl as a skeleton structure and serving as a component E, 0.0-4.34% of serial derivatives taking ester group-connected diphenyl as a skeleton structure side group difluoro-terminated cyano and serving as a component F, and 0.0-1.87% of serial derivatives taking cyclohexyl diphenyl as a skeleton structure side group difluoro-terminated fluoro and serving as a component G.
Owner:PEKING UNIV
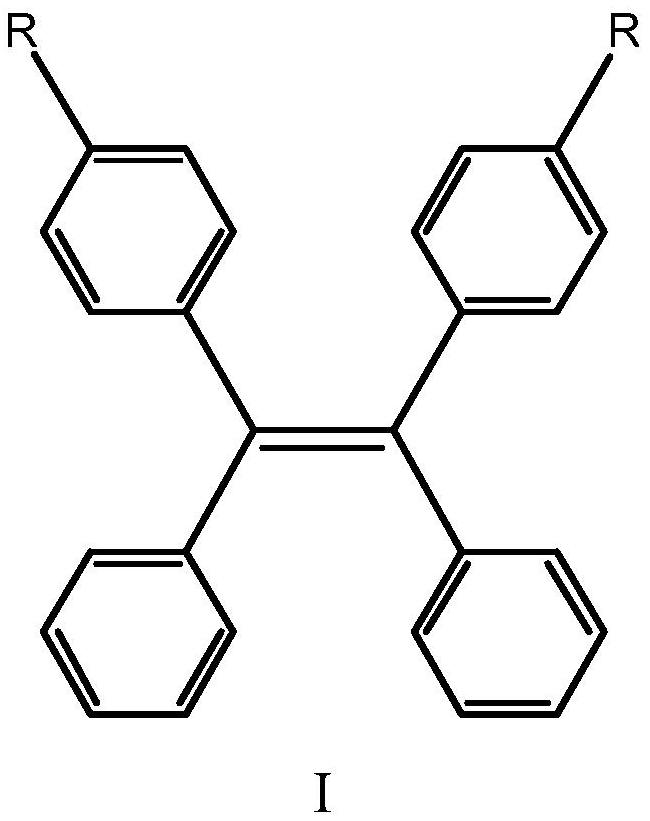
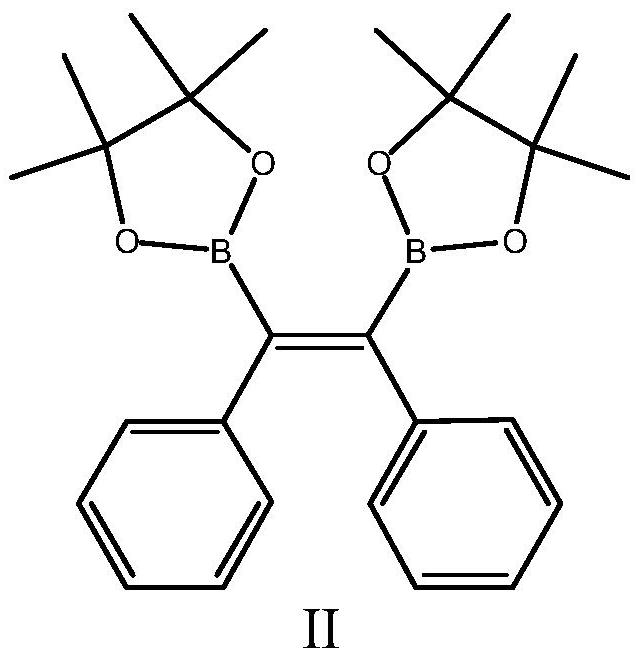
Piezochromic tetraphenylethylene compound as well as preparation method and application thereof
PendingCN113307727AStrong Molecular Aggregation-Induced Luminescent EffectSimple stepsCarboxylic acid nitrile preparationOrganic compound preparationDiphenylacetyleneBoronic acid
The invention relates to the technical field of fluorescent materials, in particular to a piezochromic tetraphenylethylene compound as well as a preparation method and application thereof. The tetraphenylethylene-based compound has excellent piezochromism and molecular aggregation-induced fluorescence, can change color without solvent fumigation and heating, and has a rapid recovery property. The preparation method comprises the following steps: reacting diphenylacetylene with bis (pinacolato) diboron to prepare stilbene (pinacolato) diboron, and reacting with a p-bromobenzene substituent to obtain a final product. Due to the piezo-chromic effect and the rapid recovery property of the piezo-chromic tetraphenylethylene compound, the piezo-chromic tetraphenylethylene compound can be applied to preparation of piezo-chromic materials at room temperature and is further applied to the fields of brain tumor imaging technologies, near-infrared probe technologies, photoelectric devices and the like.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for synthesizing diphenylacetylene by utilizing calcium carbide
InactiveCN101585749ALow costRaw materials are easy to getOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationOrganic synthesisIodide
The invention belongs to the technical field of organic synthesis intermediate, medicine and liquid crystal material, in particular, relates to a method for synthesizing diphenylacetylene by utilizing calcium carbide. The method comprises the following specific steps: adding toluene, iodobenzene, cesium carbonate, cuprous iodide, ferron, N,N'-dimethylethylenediamine, and calcium carbide into a reactor, stirring and reacting at the temperature of between 140 and 146 DEG C for 115-125 hours; water processing the reactant after finishing the reaction, extracting with ethyl acetate, washing the organic layer with saturated salt water, and drying with anhydrous sodium sulfate, and them obtaining crude product after decompression evaporating solvent; separating and purifying the crude product by column chromatography to obtain the required product. Molar ratio of calcium carbide to iodobenzene is (2-10):1; molar ratio of cesium carbonate to iodobenzene is (1.5-3):1; molar ratio of cuprous iodide to iodobenzene is (0.05-0.3):1; molar ratio of ferron to iodobenzene is (0.05-0.3):1; and molar ratio of N,N'-dimethylethylenediamine to iodobenzene is (0.05-0.3):1. The diphenylacetylene synthesized by the method can be applied to organic synthesis, liquid crystal material and pharmic synthesis intermediates. The invention takes calcium carbide as raw material and ferric chloride as catalyst. The invention has easily obtained raw material, low cost, simple technology, and easy industrialization.
Owner:TONGJI UNIV
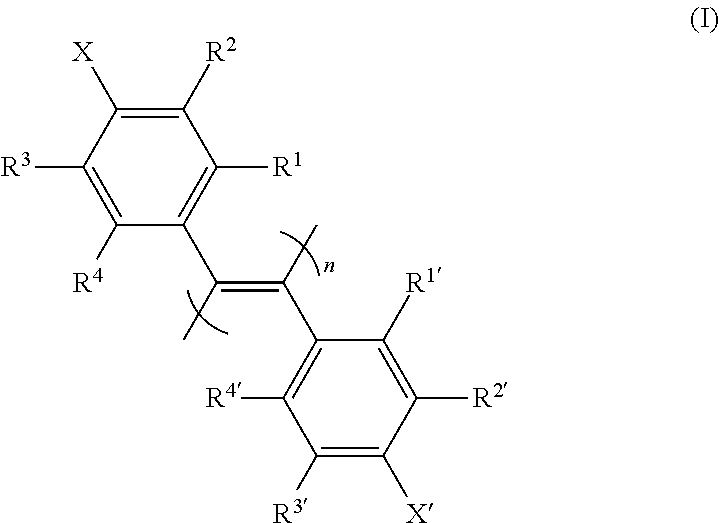
New poly(diphenylacetylene) compound, preparation method therefor, and use thereof as optical isomer separating agent
InactiveUS20150376309A1Improve abilitiesLow costSolid sorbent liquid separationChemical compoundFilling materials
The present invention provides an optical isomer separating agent and a production method thereof. That is, the present invention provides a one-handed helical poly(diphenylacetylene) compound represented by the following formula (I)[wherein each symbol is as described in the DESCRIPTION], and a production method thereof, an optical isomer separating agent containing the poly(diphenylacetylene) compound, and a packing material for a chiral column, containing the optical isomer separating agent coated on a carrier. Since these have a superior separation ability for a wide variety of compounds, a practical optical resolution method can be provided.
Owner:KANAZAWA UNIV
Side difluorine substituted diphenylacetylene liquid crystal compound, preparation method for same and application thereof
ActiveCN102633596AIncrease the number ofLarge dielectric anisotropyLiquid crystal compositionsSolid-state devicesBenzeneCrystallography
The invention discloses a side difluorine substituted diphenylacetylene liquid crystal compound, a preparation method for the same and application thereof. The compound is represented by the structural general formula I. The liquid crystal molecule performance is improved by introducing fluorine atoms into a molecular structure, increasing the molecular width and the fluorine atom number, decreasing the melting point and enhancing intermiscibility, and a molecular shape is transformed by leading two rodlike molecules including side difluorines to be in acetylenic bond linkage, so that the Z-shaped compound is formed. The side fluorine atom substituted liquid crystal compound is high in resistivity, has high dielectric anisotropy, is capable of meeting the requirement of a VA-TFT mode, and can be used as liquid crystal display materials and liquid crystal monomers. (Formula I).
Owner:BEIJING CHENGZHI YONGHUA DISPLAY TECHNOLOGY CO LTD
Bicyclohexylethylene substituted diphenylne liquid crystal compound and preparation method thereof
ActiveCN103805208BLow melting pointLow viscosityLiquid crystal compositionsHydrocarbonsCrystallographyLiquid-crystal display
The invention discloses a dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound and a preparation method thereof. The structural general formula of the dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound is shown in the specification, wherein (F)m, (F)n and (F)x respectively represent fluorine atom substitution, m, n and x represent substitution number of the fluorine atoms and the value of m, n and x is 0 or 1, and the cyclohexyl is trans-cyclohexyl; both R and R' represent C1-C15 alkyl, C1-C15 alkenyl, C1-C15 alkoxy, C1-C15 alkenyloxy, fluoro-substituted C1-C15 alkyl or fluoro-substituted C1-C15 alkenyl. The dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound not only has high clearing point, wide nematic phase section, large birefringence, and low optical absorption coefficient, but also has low melting point, low viscosity and good intermiscibility, and can be used in liquid crystal optical elements; the dicyclohexyl ethylene group substituted diphenylacetylene liquid crystal compound with side fluorine can also be used in a double frequency liquid crystal display mode.
Owner:XIAN CAIJING OPTO ELECTRICAL SCI & TECH
Hyper-branched silicon-contained aryne polymer and preparation method thereof
The invention discloses a hyper-branched silicon-contained aryne polymer and a preparation method thereof. The polymer is structurally characterized in that the polymer is a hyper-branched structure containing silicone atoms and aryne group. The preparation method of the polymer comprises the following steps: taking diethynyl-benzene, trichlorosilane or tetrachlorosilane, halohydrocarbon and magnesium powders as raw materials, and synthesizing the hyper-branched silicon-contained aryne polymer by the following three steps under the protection of inert gas: the halohydrocarbon and the magnesium powders react to generate the alkyl magnesium halide Grignard reagent firstly; the alkyl magnesium halide Grignard reagent and the diethynyl-benzene react to generate the diphenylacetylene magnesium halide Grignard reagent subsequently, and the diphenylacetylene magnesium halide Grignard reagent and the trichlorosilane or tetrachlorosilane react to generate the hyper-branched silicon-contained aryne polymer. In the invention, the process is simple, the operation is convenient, the reaction time is short, the process condition is easy to be controlled, and the post processing is simple. The prepared hyper-branched polymer is brown viscous liquid or primrose yellow powdered solid, is stable at room temperature and is easy for storage, and the condensate thereof has excellent thermal stability.
Owner:EAST CHINA UNIV OF SCI & TECH
Diphenylacetylene-containing cyclopentadienyl iron salt two-photon absorption materials and preparation method thereof
The invention relates to three cyclopentadienyl iron salt two-photon absorption materials having a diphenylacetylene group and a preparation method thereof; a diphenylacetylene structure and a cationic cyclopentadienyl iron receptor are jointly composed of a D-(pi)-A type two-photon absorption functional group, and thus the three synthesized novel cyclopentadienyl iron salts have a strong two-photon absorption ability at 532 nm, and has great application value in the two-photon photoetching field when used as the two-photon absorption type cationic photo-initiator.
Owner:BEIJING UNIV OF CHEM TECH
Process for Producing Picolinic Acid Compounds
InactiveUS20080254519A1The process is convenient and fastProduced conveniently and efficientlySugar derivativesDepsipeptidesDiphenylacetyleneDiol
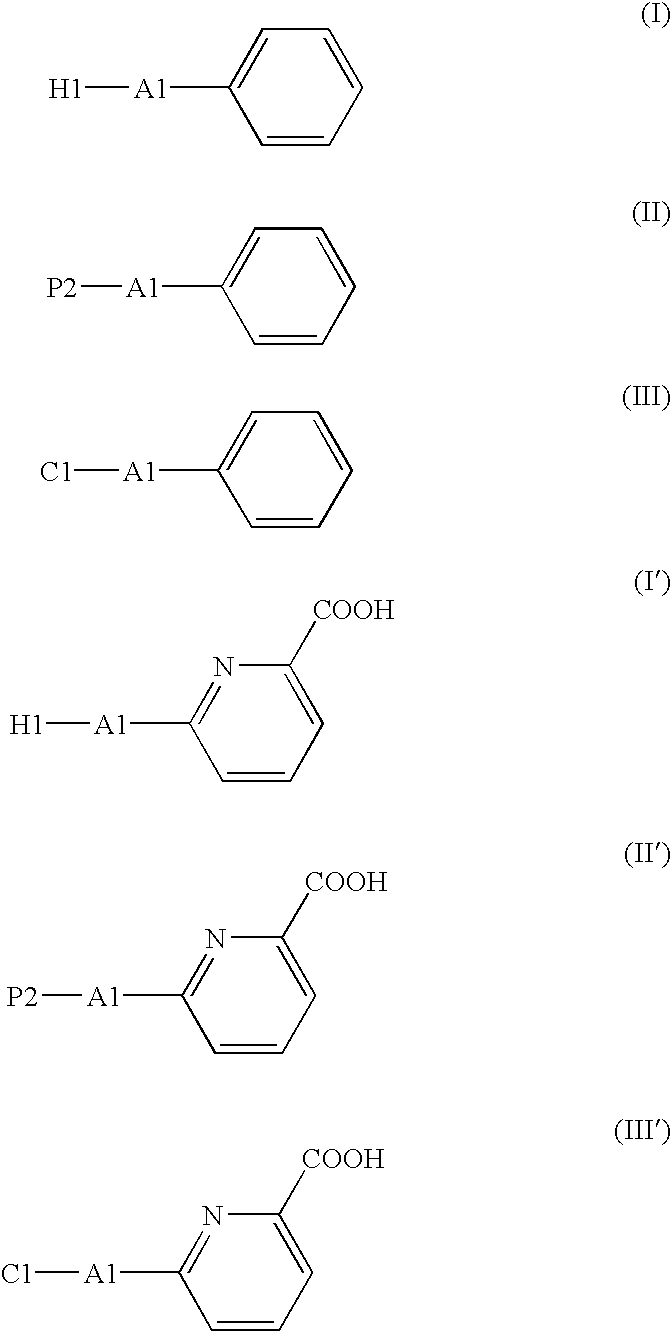
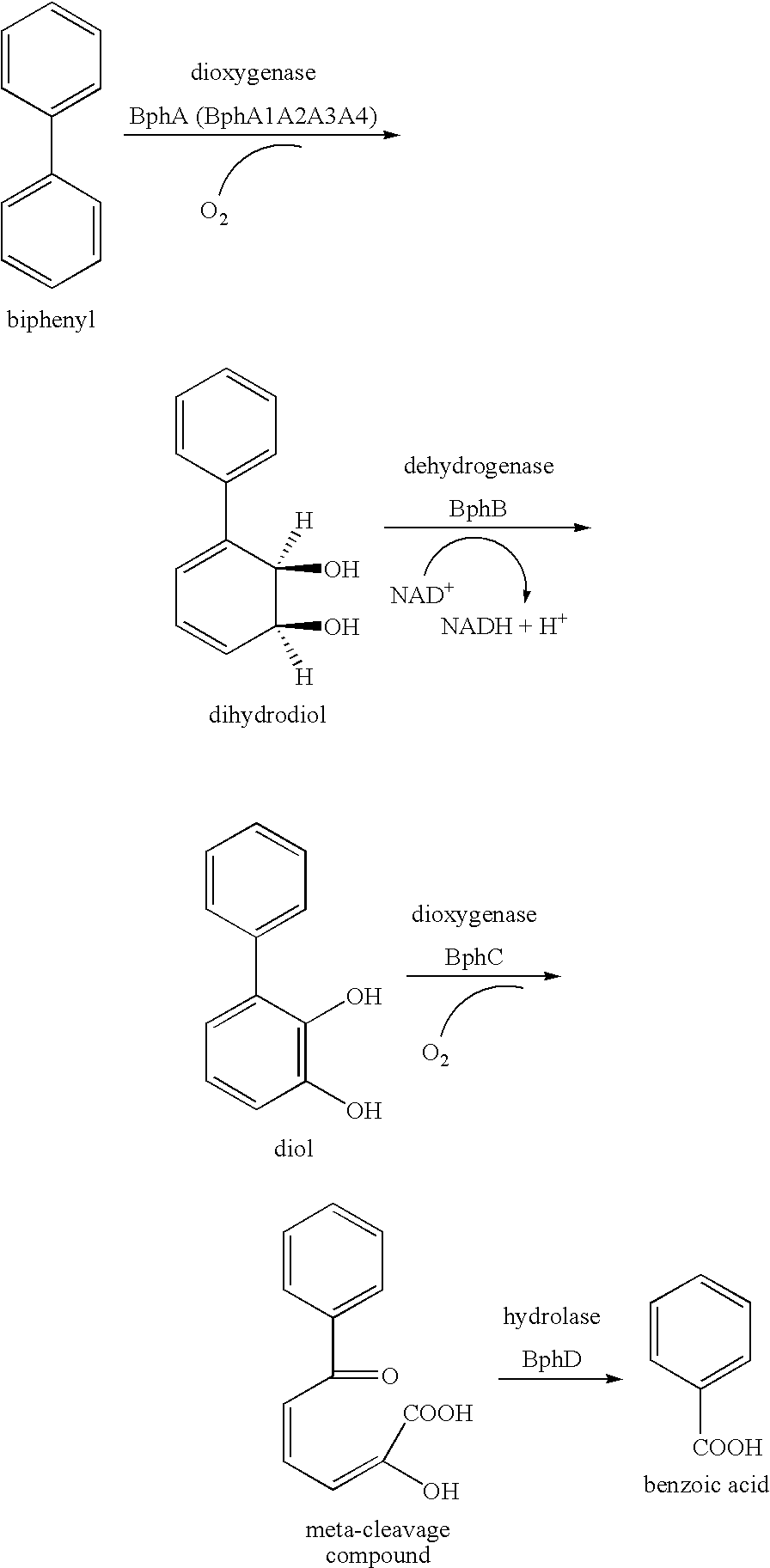
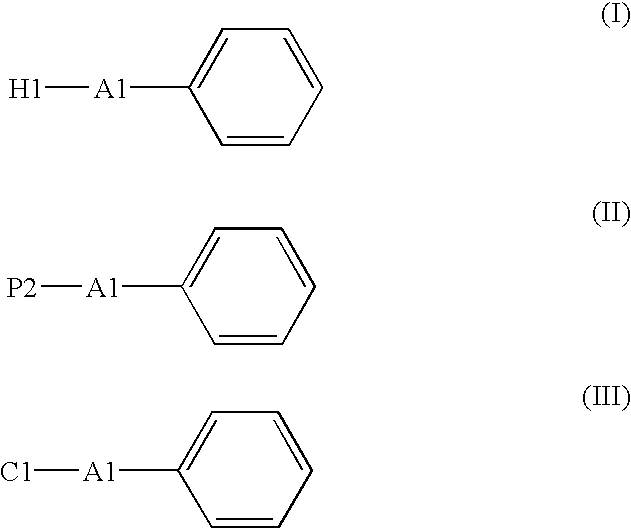
The present invention relates to a method for producing a picolinic acid compound. Specifically, the present invention relates to a method for producing a picolinic acid compound, which comprises reacting an aromatic compound that contains a phenyl group represented by the following formula (I), (II), or (III) with aromatic ring dioxygenase, aromatic ring dihydrodiol dehydrogenase, and aromatic ring diol dioxygenase, and obtaining a picolinic acid compound (I′), (II′), or (III′).wherein, H1 is an optionally substituted heterocyclic group, A1 is a single bond or an optionally substituted C1-4 alkylene group or alkenylene group, P2 is an optionally substituted phenyl group, and C1 is an optionally substituted cyclic hydrocarbon group (excluding a phenyl group), and where formula II does not represent diphenylacetylene.
Owner:SHINDO KAZUTOSHI +3
Processes for the preparation of zuclomiphene and intermediates thereof
ActiveUS11046638B2High isomeric purityHigh yieldOrganic compound preparationOrganic chemistry methodsZuclomipheneBiochemical engineering
The present invention provides processes for the preparation of zuclomiphene, as well as intermediates useful in the preparation thereof. In particular, processes are provided for the carbometallation of diphenylacetylene with a compound of Formula (3) to afford either zuclomiphene or an intermediate which is converted to zuclomiphene.
Owner:APOTEX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com