Diphenylacetylene-containing cyclopentadienyl iron salt two-photon absorption materials and preparation method thereof
A two-photon absorption, cyclopentadienyl iron technology, applied in the directions of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor resin compatibility and complex synthesis process, and achieve a simple and good preparation process. The effect of compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
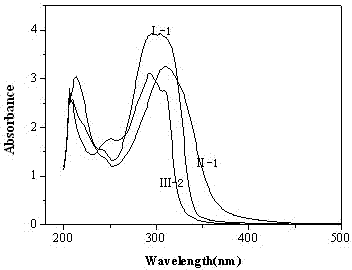
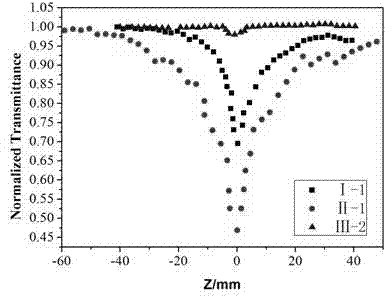
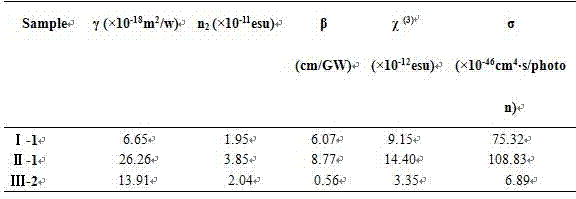
Image
Examples
Embodiment 1
[0044]In the invention, the initial substance is p-bromoaniline, the intermediate product p-bromoiodobenzene is prepared by diazotization reaction, and then it is connected with phenylacetylene by Sonogashira coupling reaction to obtain another intermediate product bromotoluene; with 4- Bromophenol is prepared as a raw material to obtain 4-hydroxyphenylboronic acid, which is then reacted with brominated tolanylacetylene by Suzuki coupling to obtain 4-(phenylethynyl) biphenol, which is then combined with [( n 6 - 1,4 - Dichlorobenzene)-iron-( n 5 - Cyclopentadiene)] hexafluorophosphate reaction to obtain [( n 6 -1,4-bis(phenylethynylbiphenoxy)benzene)-iron-( n 5 -cyclopentadiene)] hexafluorophosphate. Concrete synthetic route is shown as follows:
[0045]
[0046] In the formula:
[0047] a: NaNO 2 , HCl, 0-5°C, react for 0.5 hours, KI;
[0048] B: ditriphenylphosphine palladium dichloride, triphenylphosphine, cuprous iodide;
[0049] c: TBDMSCl, Mg, THF, B(...
Embodiment 2
[0070] In the invention, 4-(2-phenylethynyl)-bromobenzene (synthetic method is the same as in Example 1) is utilized to prepare 4-(2-phenylethynyl) phenylboronic acid, and then reacted with [(η 6 -1,4-dichlorobenzene)-iron-(η 5 -Cyclopentadiene)] hexafluorophosphate is connected to obtain the target compound [( n 6 -1,4-diphenylethynylbiphenyl)-iron-( n 5 -cyclopentadiene)] hexafluorophosphate. Concrete synthetic route is shown as follows:
[0071]
[0072] (1) Preparation of 4-phenylacetylene boronic acid
[0073]
[0074] In a 100ml three-neck flask, add 2g (7.78mmol) of 4-(2-phenylethynyl)-bromobenzene and 30ml of tetrahydrofuran, start stirring, protect with nitrogen to dissolve, and cool down to -78°C with an acetone-liquid nitrogen bath. Add 5.3ml (8.5mmol) of 1.6M n-butyllithium cyclohexane solution dropwise to the system, keep at -78°C, react for 0.5 hours, and add 1.21g (11.67mmol) trimethyl borate dropwise to the system. After the dropwise addition, th...
Embodiment 3
[0080] Same as the preparation method of Example 1, just change the reactant into p-iodophenol and 4-methoxyphenylacetylene to react to prepare 4-(4-methoxy)phenylacetylene phenol, and [( n 6 - 1,4 - Dichlorobenzene) -Iron-( n 5 - cyclopentadiene)] hexafluorophosphate to obtain the target product [( n 6 -1,4-bis((4-methoxyphenylethynyl)phenoxy)benzene)-iron-( n 5 -cyclopentadiene)] hexafluorophosphate. Concrete synthetic route is shown as follows:
[0081]
[0082] (1) Preparation of 4-methoxyphenylacetylene
[0083] Add NaOH 4.16g (0.104mol) and water 17ml into a 250ml three-necked flask, heat to reflux, and dropwise add ( Z )-3-Chloro-3-(4-methoxyphenyl)acrolein 19.6g (0.1mol) in 1,4-dioxane solution, the dropwise addition was completed, and the reaction was carried out for 4 hours, and the reaction progress was tracked by TLC. After the reaction was completed, cool to room temperature, stand to separate the layers, separate the organic phase, extract th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com