Synthetic method of phenytoin sodium
A synthetic method, the technology of phenytoin sodium, applied in the field of biomedicine, can solve the problems of reducing the yield of phenytoin, low yield of phenytoin, and low purity, so as to inhibit the formation of by-product diphenylacetylene diurea and promote the process of amidation reaction , the effect of reducing the reaction concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
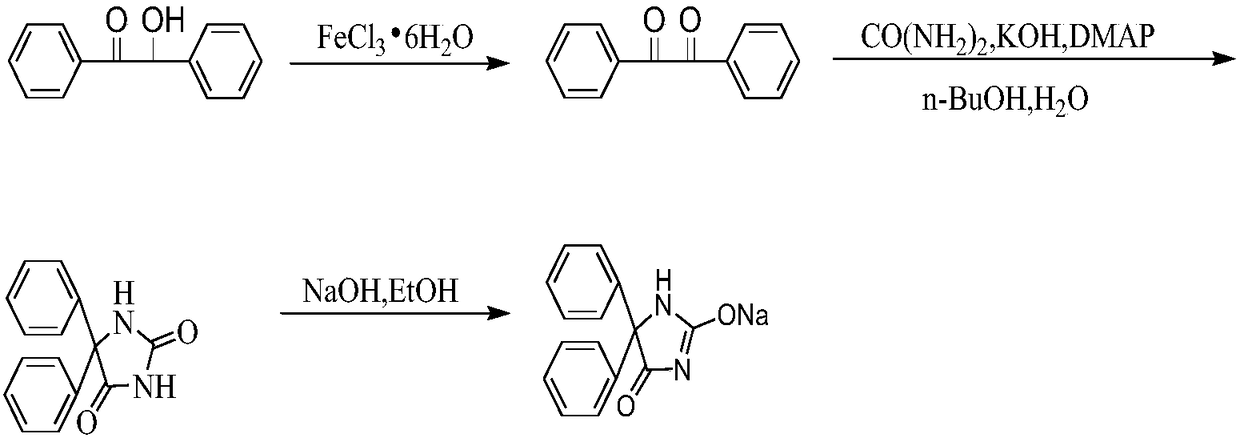
[0032] Oxidation reaction: Weigh 2.18g of benzoin, 5.4g of ferric chloride hexahydrate, measure 13mL of glacial acetic acid, and 5.2mL of distilled water, pour them into a three-necked flask in turn, stir, heat and reflux for 50min, and stop heating completely after the reaction; after cooling, put Pour the reaction solution into a 100mL beaker, add 40mL of distilled water, and bathe in ice-salt for 15min, wait for the precipitation of yellow needle-like crystals, filter with suction, wash the filter cake with distilled water several times, and obtain 2.14g of dibenzoyl;
[0033] Condensation reaction: Add 2.14g dibenzoyl, 1.80g urea, 2.13g potassium hydroxide, 0.06g 4-dimethylaminopyridine, 22mL n-butanol, 22mL distilled water into a 250mL three-neck round bottom flask, stir magnetically, and Reflux at ℃ for 30 minutes, stop heating completely; cool the reaction solution to room temperature, pour into a 500mL separatory funnel, extract 3 times with 3×100mL distilled water, com...
Embodiment 2
[0036] Oxidation reaction: Weigh 8.67g of benzoin, 24.5g of ferric chloride hexahydrate, measure 50mL of glacial acetic acid, and 22mL of distilled water, pour them into a three-neck flask in turn, start stirring, and heat to reflux for 52 minutes, then stop heating completely; after cooling, Pour the reaction solution into a 500mL beaker, add 173mL of distilled water, and bathe in ice-salt for 16 minutes, yellow needle-like crystals precipitate, filter with suction, and wash the filter cake with distilled water several times to obtain 8.5g of dibenzoyl;
[0037] Condensation reaction: Add 8.5g of dibenzoyl, 7.4g of urea, 9g of potassium hydroxide, 0.26g of 4-dimethylaminopyridine, 71mL of n-butanol, and 85mL of distilled water into a 250mL three-necked round-bottomed flask, stir magnetically, and heat to reflux at 118°C 35min, stop heating after the reaction is complete. The reaction solution was cooled to room temperature, poured into a 500mL separatory funnel, extracted 3 t...
Embodiment 3
[0040] Oxidation reaction: Weigh 10.71g of benzoin, 28g of ferric chloride hexahydrate, measure 64mL of glacial acetic acid, and 25mL of distilled water, pour them into a three-neck flask in turn, start stirring, heat and reflux for 55min, and stop heating completely after the reaction; after cooling, the reaction Pour the solution into a 500mL beaker, add 210mL distilled water, and ice-salt bath for 18 minutes, yellow needle-like crystals precipitated, filtered with suction, and washed the filter cake with distilled water several times to obtain bibenzoyl;
[0041] Condensation reaction: Add 10.5g dibenzoyl, 9g urea, 10.64g potassium hydroxide, 0.305g 4-dimethylaminopyridine, 105mL n-butanol, 125mL distilled water into a 500mL three-neck round bottom flask, stir magnetically, and heat to 119 ℃ reflux for 40min, stop heating after the reaction is complete. The reaction solution was cooled to room temperature, poured into a 500mL separatory funnel, extracted 3 times with 3×100m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com