Patents
Literature
30 results about "Phenytoin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
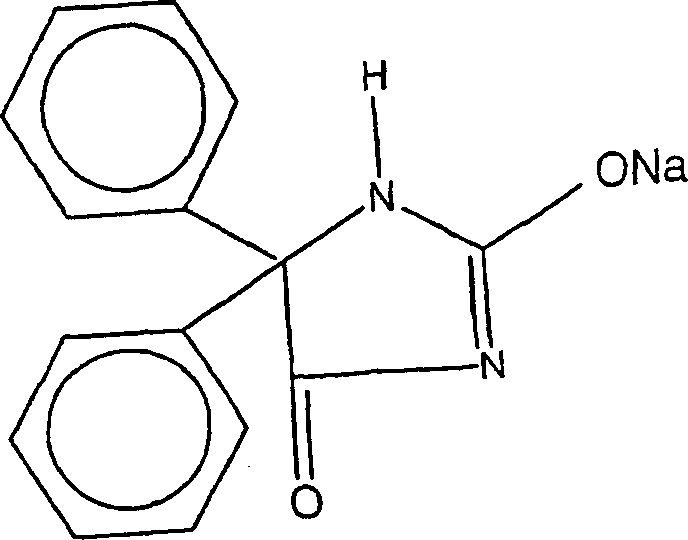
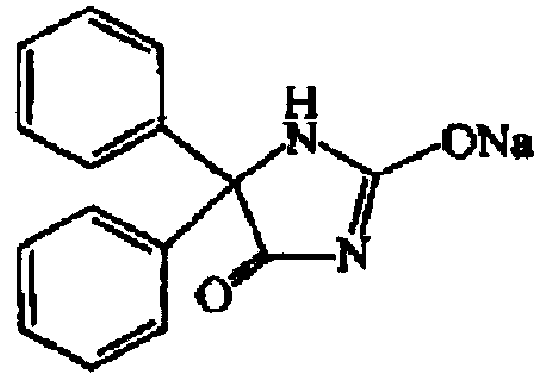
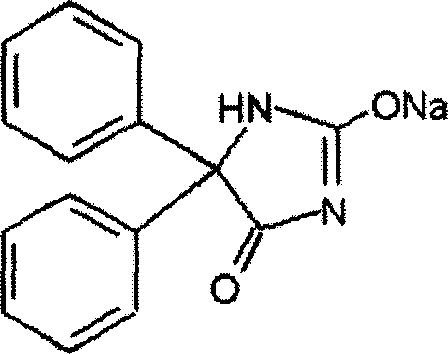
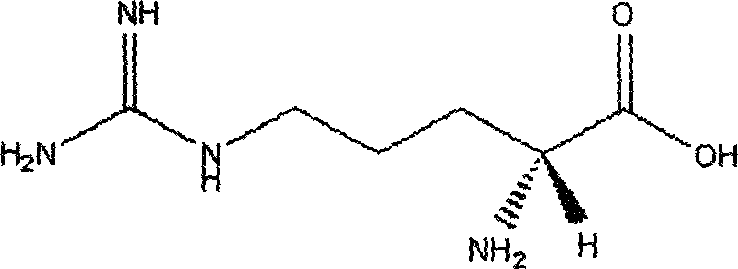
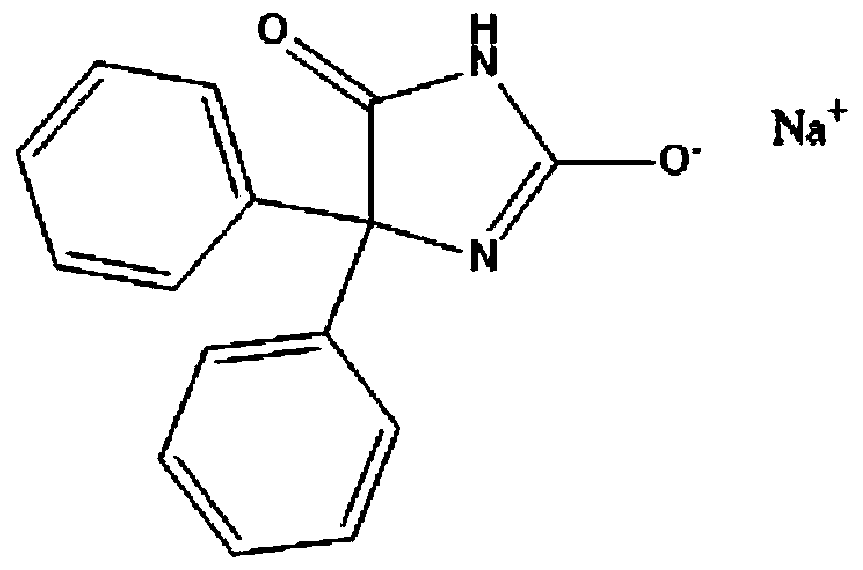
The sodium salt form of phenytoin, a hydantoin derivate and non-sedative antiepileptic agent with anticonvulsant activity. Phenytoin sodium promotes sodium efflux from neurons located in the motor cortex, thereby stabilizing the neuron and inhibiting synaptic transmission. This leads to a reduction in posttetanic potentiation at synapses, an inhibition of repetitive firing of action potentials and ultimately inhibits the spread of seizure activity.
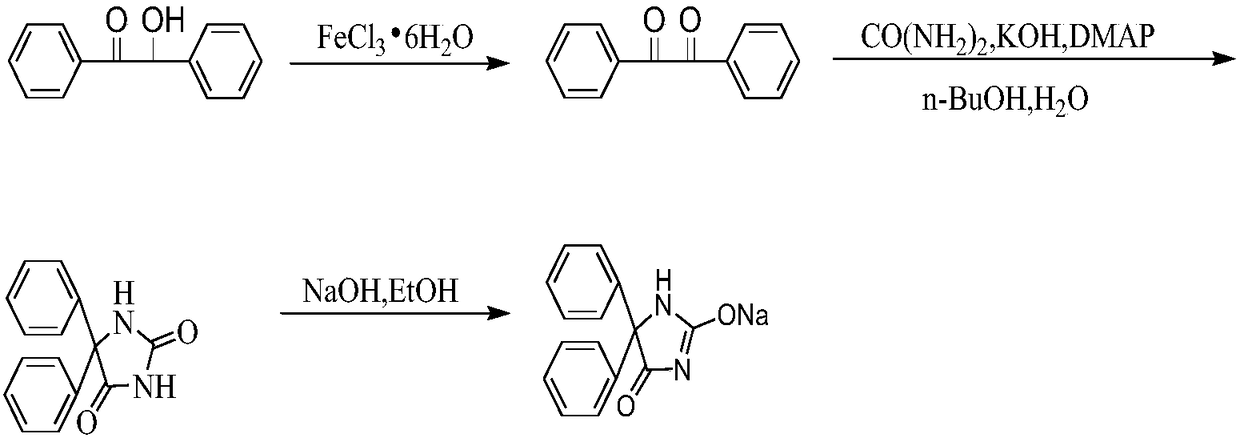
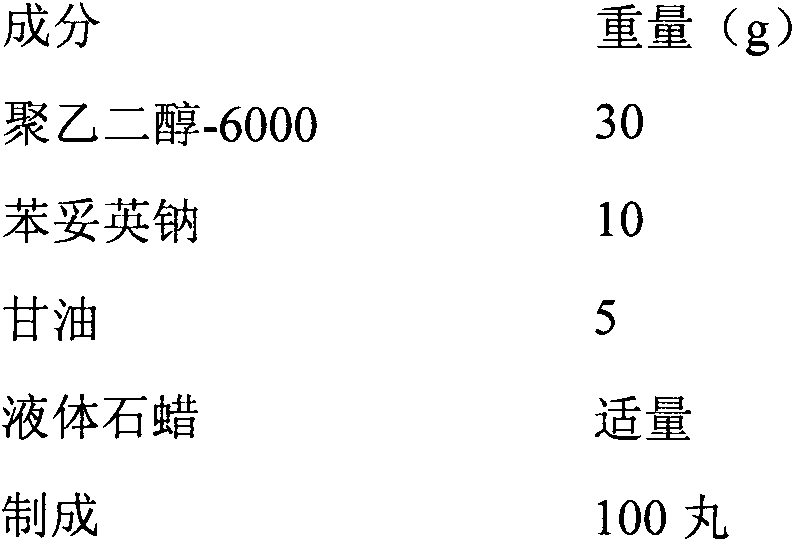
Synthetic method of phenytoin sodium
ActiveCN109456271AReduce the reaction concentrationSuppress generationOrganic chemistryDiphenylacetyleneN-Butanol
The invention relates to a synthetic method of phenytoin sodium, belonging to the technical field of biomedicine. The synthetic method of the phenytoin sodium comprises the following steps of oxidation reaction, condensation reaction and salt formation reaction, wherein a amidation reaction process is promoted through adding a phase transfer catalyst 4-dimethylamiopryidine in the condensation reaction; a two-phase system of n-butanol and water is adopted, so that the generation of diphenylacetylene diurea is greatly inhibited, and reaction time is obviously shortened; ethanol serves as a solvent in the salt formation reaction, after a sodium hydroxide ethanol solution reacts with phenytoin, cyclohexane with defective solvent ice is added to promote the phenytoin sodium to precipitate as white crystal, and compared with the salt formation reaction with water as a solvent, the phenytoin sodium is faster in precipitation rate and high in precipitation degree and purity.
Owner:NINGBO POLYTECHNIC
Pharmaceutical composition for extended release of phenytoin sodium
InactiveUS20060034910A1Easy to operateCapsule deliveryGranular deliveryEngineeringPharmaceutical medicine
The present invention provides an oral extended release solid pharmaceutical composition of phenytoin sodium or its pharmaceutically acceptable derivative thereof and the process of manufacturing the same the said extended release oral solid pharmaceutical composition comprising of at least one suitable pharmaceutically acceptable excipient alongwith phenytoin sodium. The said extended release oral solid pharmaceutical composition is manufactured by blending phenytoin sodium with at least one suitable pharmaceutically acceptable excipient and granulating the blend optionally using at least one suitable binder dissolved in an organic solvent or mixture of solvents.
Owner:WOCKHARDT LTD
Rapid detection method for phenytoin sodium
InactiveCN106153954AHigh potencyHigh sensitivityBiological testingPretreatment methodCarrier protein
The invention discloses a rapid detection method for phenytoin sodium. A phenytoin sodium antigen and an antibody which are prepared by virtue of the rapid detection method have relatively good specificity. A phenytoin sodium coupled carrier protein is selected from hemocyanin KLH or bovine serum albumin BAS. By virtue of immunization of a mouse by virtue of the antigen, the obtained phenytoin sodium antigen has relatively high valence and relatively good sensitivity. By utilizing a prepared phenytoin sodium detection card, the detection speed is high, a result is easily observed, and the rapid screening of batch samples can be realized, so that the detection cost can be greatly lowered, the workload is reduced, and the phenytoin sodium detection card has practical significance. According to the rapid detection method, aiming at solving the problem that a to-be-detected sample has complex components, a sample pretreatment method is optimized, the required sample amount is low, and the sample pretreatment can be finished only through two steps including extraction and dilution, so that the detection university and the detection efficiency are greatly improved.
Owner:广东省药品检验所
An alepsin slow-releasing gel for promoting paradontal part reborn as well as preparation method and application
InactiveCN101156849AHigh and long-lasting drug concentrationSave human effortOrganic active ingredientsDigestive systemSustained release drugMicrosphere
The invention discloses phenytoin sodium sustained-release gel which promotes periodontal regeneration, and the phenytoin sodium sustained-release gel is made of components of phenytoin sodium, PLGA sustained-release microspheres and poloxamer gel, wherein, the content of an active component, the phenytoin sodium is 2 to 5 mg / ml. The preparation method of the phenytoin sodium sustained-release gel which promotes the periodontal regeneration has the steps that firstly, the PLGA microspheres containing the phenytoin sodium is prepared in W / O / W type emulsified solvent volatilization method; secondly, the microspheres are mixed into 25 percent of the poloxamer gel, to produce microsphere gel containing 2 to 5 mg / ml phenytoin sodium. The invention provides a new safe, effective drug with acceptable price and easy promotion and application for local administration of periodontal disease therapy. The preparation method of the phenytoin sodium sustained-release gel provided by the invention is convenient, and has strong operability, the prepared sustained-release drug has stable performance, and the distribution of the microsphere graininess is more uniform.
Owner:SHANDONG UNIV
Sustained release preparation of phenytoin sodiumslow release
InactiveCN101015532AImprove securityImprove effectivenessOrganic active ingredientsInorganic non-active ingredientsEnteral administrationBlood concentration
The invention discloses a slow release preparation of Phenytoinum Natricum and its preparing process, wherein the raw materials of the invention include Phenytoinum Natricum of a predetermined proportion, slow release matrix material and medicinal material, the preparation can be prepared into solid dispersing agent, wherein the medicament can be released slowly and continuously after being administrated, the effective concentration in blood can be maintained, and long action can be achieved. The advantages of the invention include decreased frequency of medicinal administration, improved patient's adaptability, lowered blood concentration peak-valley, increased medicinal effect and safety, and reduced total medicinal dose, thereby optimum curative effect can be achieved through minimum dose, thus the preparation is more suitable for patients.
Owner:刘凤鸣
Anti-epileptic medicine composition and preparation method thereof
InactiveCN104147020AEase of efficacySignificant effectNervous disorderHeterocyclic compound active ingredientsSide effectSilicic acid
The invention belongs to the technical field of medicines and preparation, in particular relates to an anti epileptic medicine composition and a preparation method thereof, the specific ratio is as follows: the weight ratio of aspirin to caffeine to phenobarbital sodium to phenytoin sodium is 1-5:1-2:1-6:3-25; the preparation method is as follows: adding medicinal starch and magnesium stearate in the mixture, stirring evenly, and processing to obtain a capsule; or adding lactose, microcrystalline cellulose, carboxymethyl cellulose formaldehyde calcium and silicic acid anhydride into the mixture for mixing evenly, and then adding carboxyl propionyl cellulose dissolved with isopropanol, mixing evenly, using a wet method for granulation, drying, finishing, and mixing with the magnesium stearate for tabletting to obtain a tablet; the anti epileptic medicine composition has the advantages of simple composition, easily obtained raw materials, simple preparation, remarkable curative effect, no toxic side effect and safety and reliability.
Owner:QINGDAO ZHONGREN PHARMA
Extended release pharmaceutical composition of phenytoin sodium
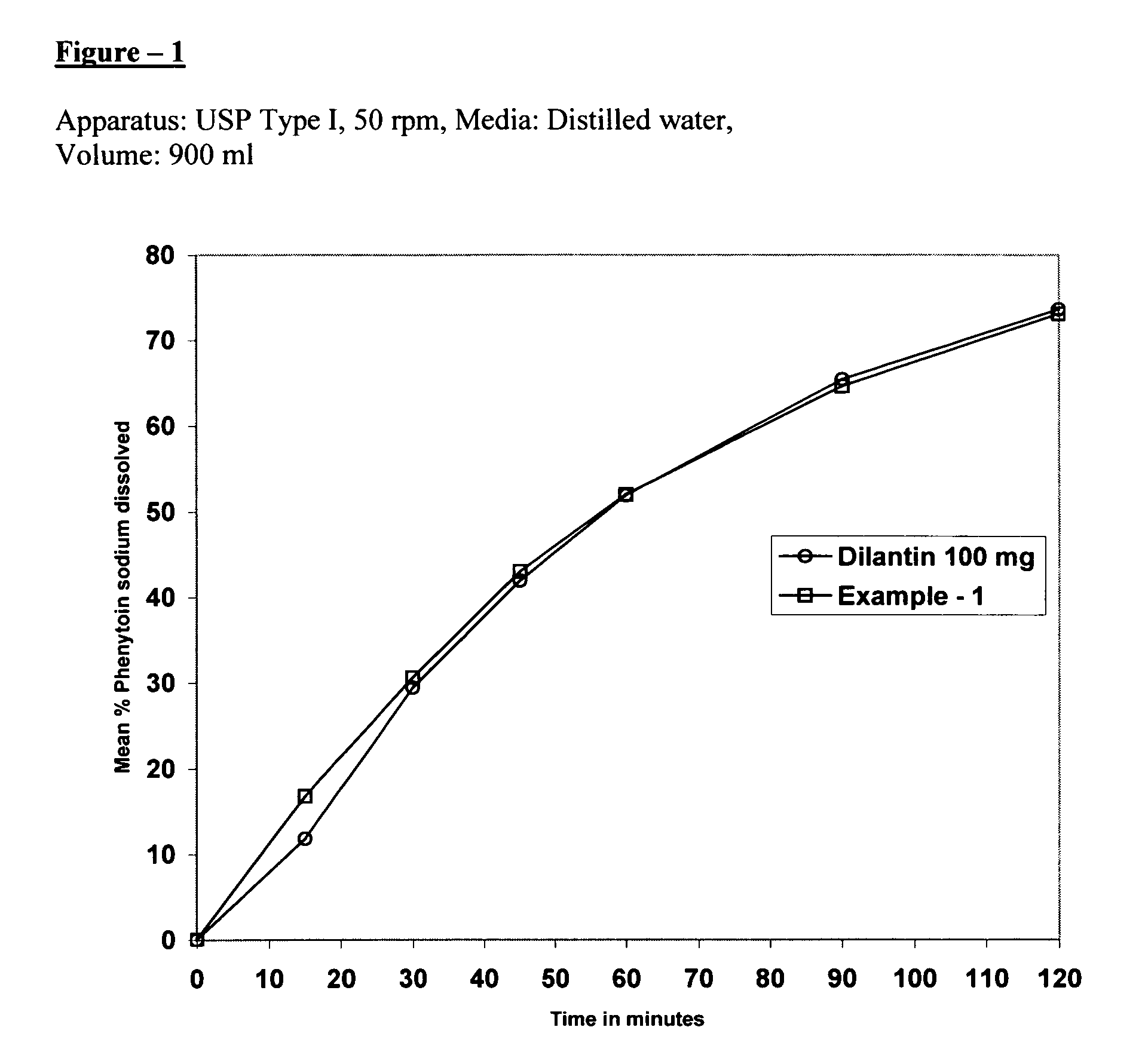
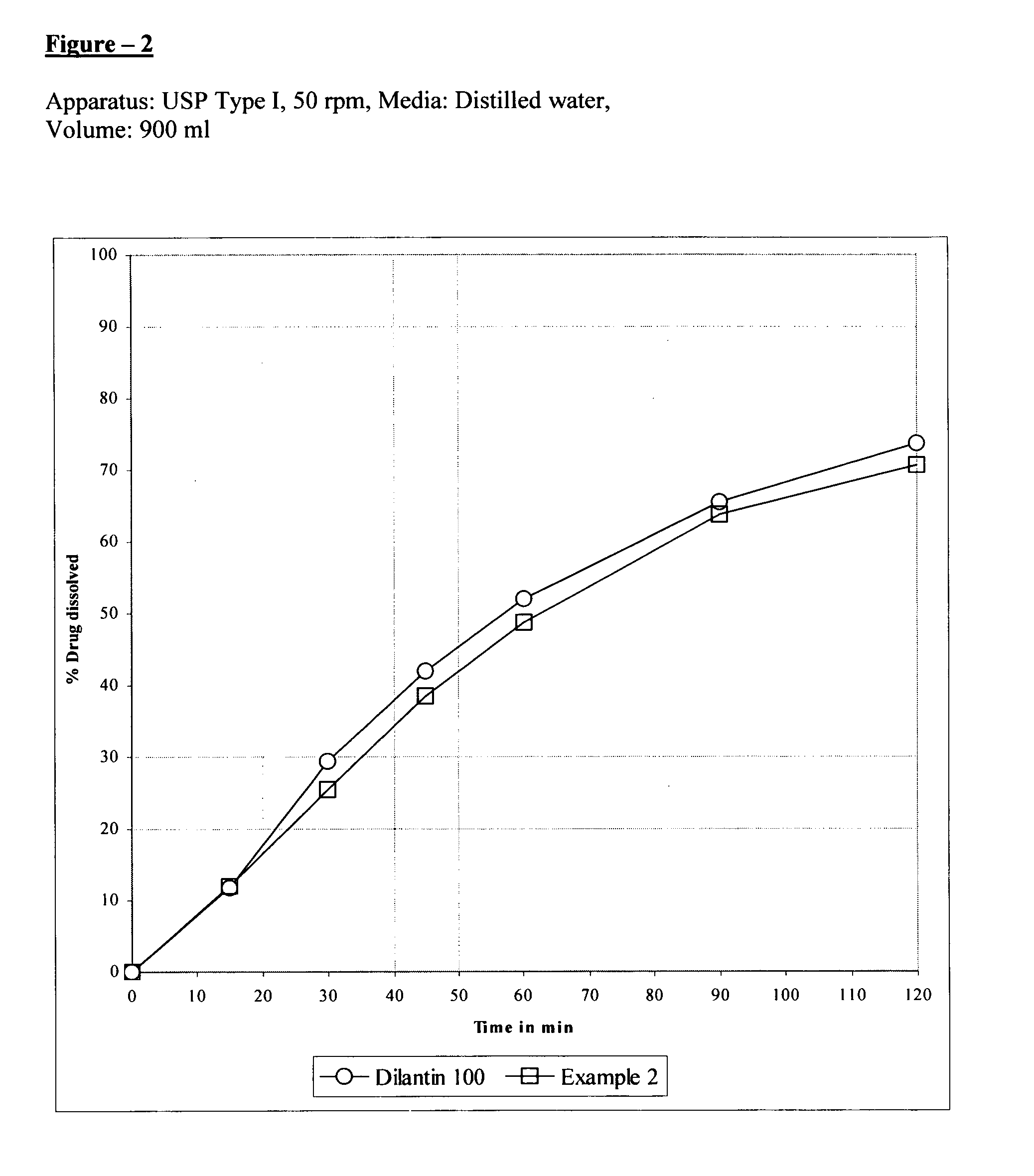
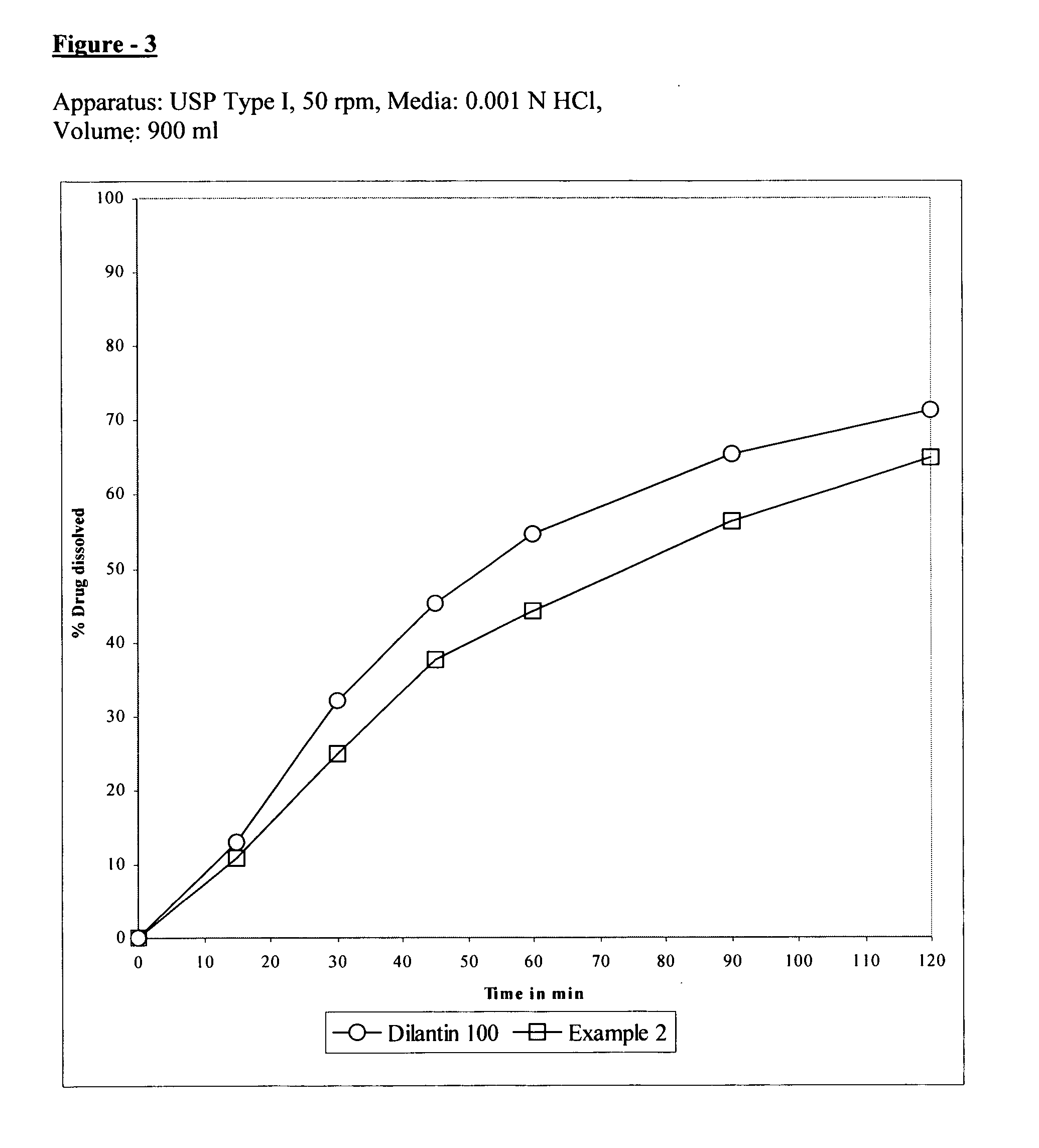
The present invention relates to extended release pharmaceutical composition of phenytoin sodium that includes a blend of phenytoin sodium and one or more hydrophilic polymers. The blend forms a matrix after contacting an aqueous media and the matrix retains at least about 20% of the phenytoin after 1 hour. It also relates to a process for preparing the extended release pharmaceutical composition.
Owner:RANBAXY LAB LTD
Application of phenytoin sodium in preparing anti-angiogenesis medicine
InactiveCN103251588AGrowth inhibitionOrganic active ingredientsSenses disorderHuman cancerCancer cell
The invention relates to an application of phenytoin sodium in preparing an anti-angiogenesis medicine. Reports related to the anti-angiogenesis activity of phenytoin sodium are unavailable so far. The invention provides an application of phenytoin sodium in preparing an anti-angiogenesis medicine, an anti-tumor medicine and an anti-wet senile vision macular degeneration medicine. An in-vivo pharmacodynamic experiment adopting a zebra fish angiogenesis model proves that the phenytoin sodium can obviously inhibit the zebra fish angiogenesis and the growth of transplanted human cancer cells, and has a therapeutic effect on wet senile vision macular degeneration. Therefore, the phenytoin sodium can be used for preparing a medicine for resisting angiogenesis, tumor or wet senile vision macular degeneration.
Owner:HANGZHOU LEISUO PHARMA
Compaction process for manufacture of sodium phenytoin dosage form
A process for the roller compaction and manufacture of a pharmaceutical formulation comprises the steps of adding sodium phenytoin to a vessel of a blender and adding at least one excipient to the vessel. The mixture is blended and transferred to a roller compactor, where pressure is applied to the blend of sodium phenytoin and excipient. Next, the resultant compaction is milled to form a granulation, which is blended a second time and is suitable for further processing into a dosage form. Preferably, the excipients include magnesium stearate, sugar, lactose monohydrate, and talc. In an alternative embodiment, talc is added immediately prior to the granulation being blended for a second time.
Owner:WARNER-LAMBERT CO
Biodegradable anastomosis nail and preparation method thereof
The invention relates to the technical field related to medical instruments, and provides a biodegradable anastomosis nail. The biodegradable anastomosis nail comprises a degradable nail body, and an anti-inflammatory and antibacterial layer is arranged on the outer wall of the degradable nail body; the degradable nail body is made of degradable magnesium alloy containing Ag or degradable zinc alloy containing Ag; the anti-inflammatory antibacterial layer comprises an anti-inflammatory mixture, and the anti-inflammatory mixture comprises phenytoin sodium and a silver ammonia solution. The anastomosis nail is set to be made of Ag-containing degradable magnesium alloy or Ag-containing degradable zinc alloy, so that the anastomosis nail for intestinal anastomosis can be degraded in vivo through microorganisms, and the arranged anti-inflammation antibacterial layer can further play roles in inhibiting bacteria, sterilizing and inhibiting infection when a same anastomosis nail is degraded, so that the problems of chronic infection, chronic inflammation and the like caused by the anastomosis nail are reduced. The problems of the chronic infection, the chronic inflammation, pain, foreign body sensation and the like caused by remaining of the anastomosis nail in the body after an operation are solved.
Owner:THE SIXTH AFFILIATED HOSPITAL OF SUN YAT SEN UNIV +1
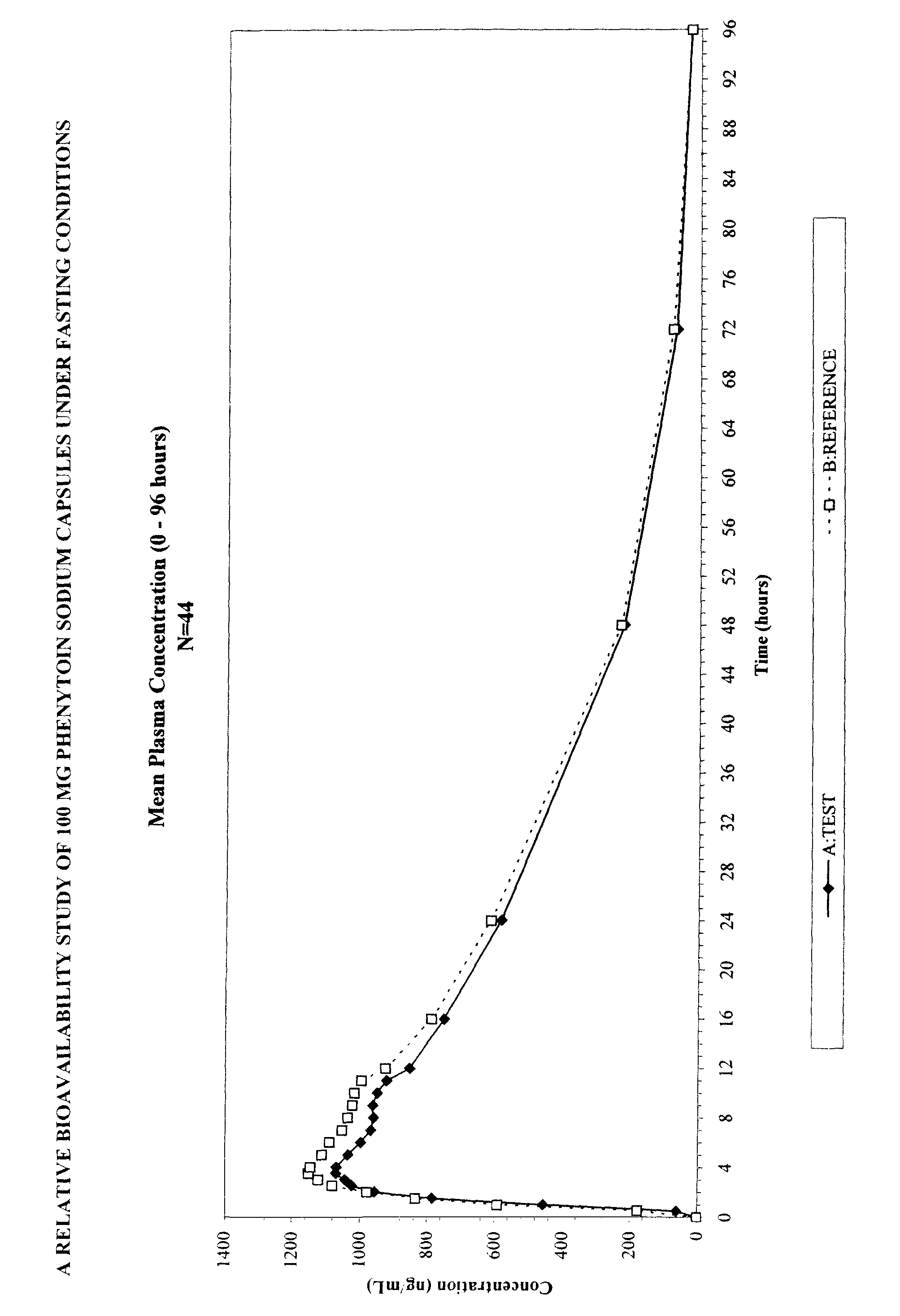
Extended release, multiple unit dosage forms of phenytoin sodium and processes for their preparation
The present invention relates to oral extended release, multiple unit dosage forms of phenytoin sodium in which individual units comprising phenytoin sodium are coated with one or more film forming polymers. The individual units include between greater than 75% w / w and about 90% w / w of phenytoin sodium.
Owner:MURPANI DEEPAK +1
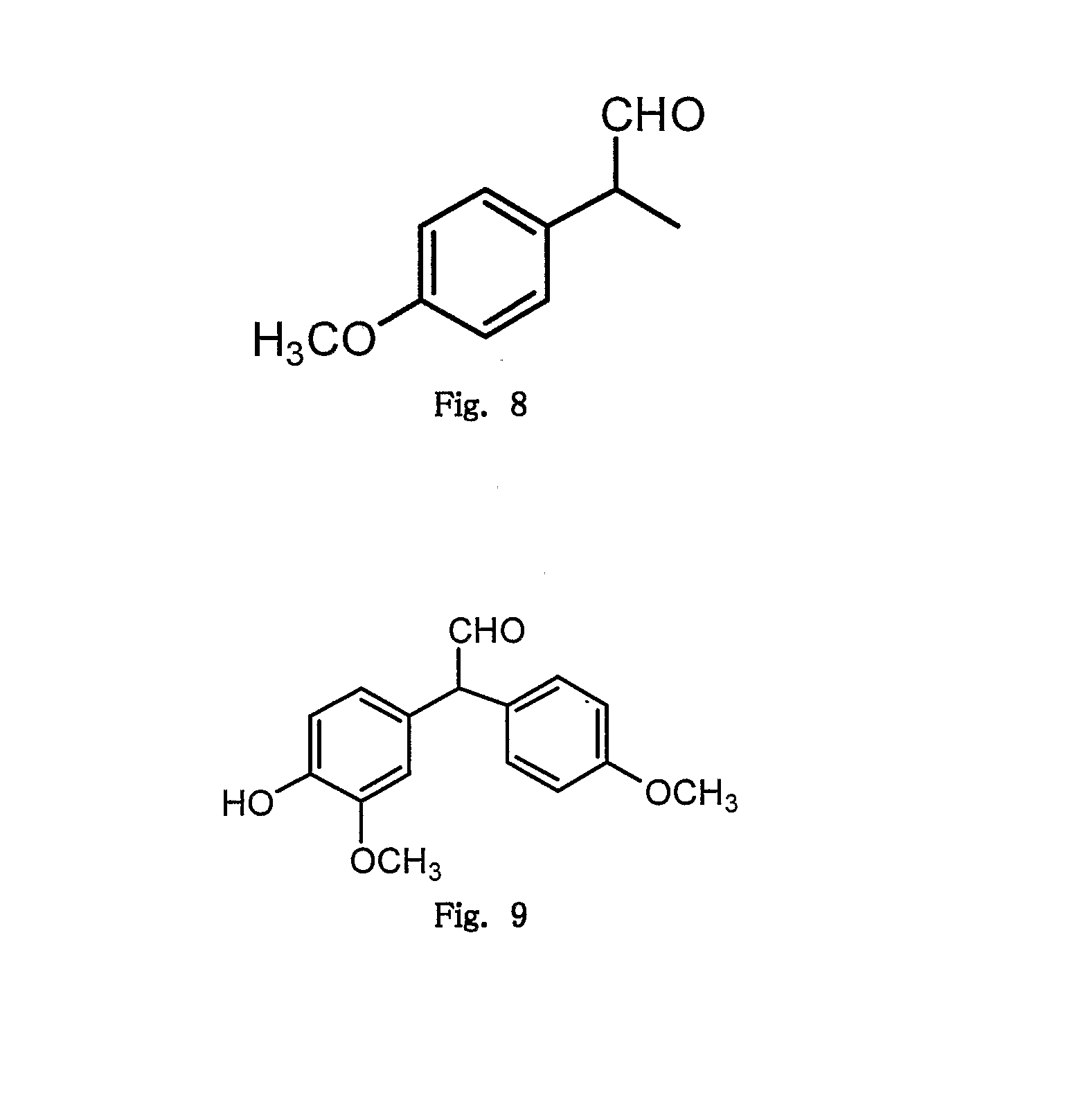
Microwave induced single step green synthesis of some novel 2-aryl aldehydes and their analogues
InactiveUS20120041234A1Potent anticancer activityOrganic compound preparationCarbonyl compound preparation by oxidationNonsteroidal Antiinflammatory Drugs/NSAIDsPhenstatin
The present invention provides a process for the preparation of some novel 2-aryl and 2,2-diaryl aldehydes and analogues which are privileged intermediates for commercially important nonsteroidal anti-inflammatory drugs including naproxen, flurbiprofen and potent anticancer drug candidates, including phenstatin through a unique single step synthetic methodology utilizing easily available substrates in the form of aryl alkenes as well as environmentally benign aqueous reaction conditions in the form of solvents such as mixtures of water and DMSO or Dioxane and reagents N-bromosuccinimide, N-iodosuccinimide, N-cholorosuccinimide and phase transfer catalyst such as cetyltrimethyl ammonium bromide, N-hexyl ammonium chloride for a reaction time varying from 1 min-30 min, depending upon microwave or conventional heating, without using expensive transition metal catalysts or lewis acids / bases with yield varying from 35-55%, depending upon the solvent and substrate used. The developed method provides a clean and convenient alternative to access a diverse range of medicinally important 2-aryl and 2,2-diaryl aldehyde based scaffolds in lieu of the conventional multistep protocols employing expensive and hazardous transition metal catalysts and lewis acids / bases.
Owner:COUNCIL OF SCI & IND RES
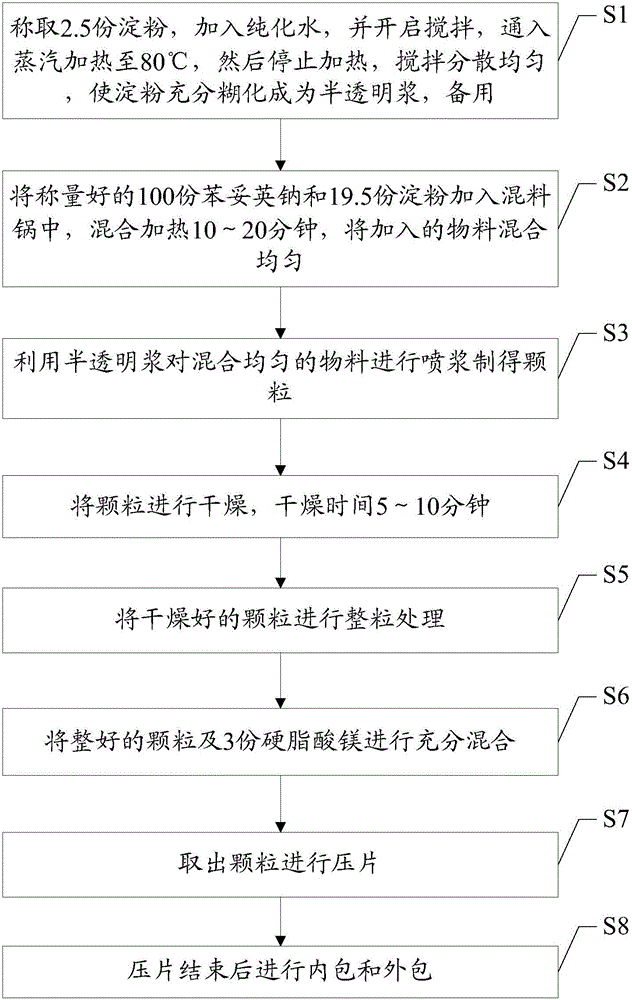
Pharmaceutical composition containing phenytoin sodium compound as well as preparation method of composition
InactiveCN105012265AGood effectImprove stabilityOrganic active ingredientsNervous disorderMagnesium stearatePhenytoin Sodium
The invention discloses pharmaceutical composition containing a phenytoin sodium compound as well as a preparation method of the composition. The pharmaceutical composition containing the phenytoin sodium compound comprises, in parts by mass, 100 parts of phenytoin sodium, 22 parts of starch and 3 parts of magnesium stearate. The pharmaceutical composition which contains the phenytoin sodium compound and is prepared with the preparation method is good in efficacy and good in stability, and the preparation cost is low.
Owner:SHANGHAI WORLDBEST ANHUI JINHUI PHARMA CO LTD
Medicine composition for treating epilepsy by combined treatment of traditional Chinese medicine and western medicine
The invention discloses a medicine composition for treating epilepsy by combined treatment of traditional Chinese medicine and western medicine, comprising active ingredients and pharmaceutic adjuvant. The active ingredients comprise traditional Chinese medicines and western medicines. The traditional Chinese medicines comprise rhizoma cyperi, gambir plant, white silkworm, radix gentiana, radix scutellariae, blackend swallowwort root, radish seed, rhizoma arisaematis, root of pilose asiabell, bighead atractylodes rhizome, fructus aurantii, haematitum, amber and rhizoma acori graminei. The western medicines are diazepam, carbamazepine and phenytoin. The medicine composition disclosed by the invention is convenient for taking and has outstanding curative effects, does not relapse after recovery and has cure rate of more than 98%.
Owner:王爱华
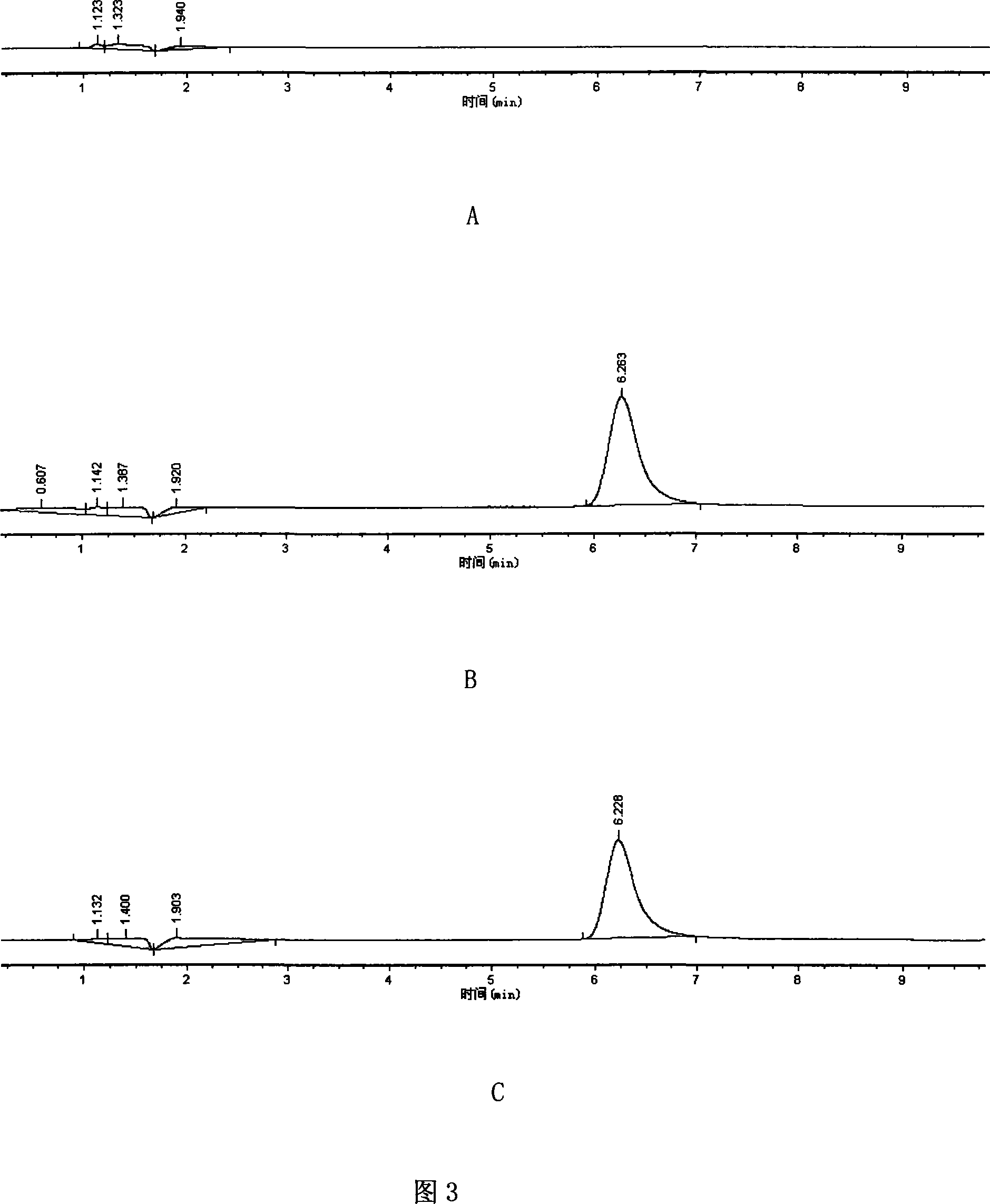
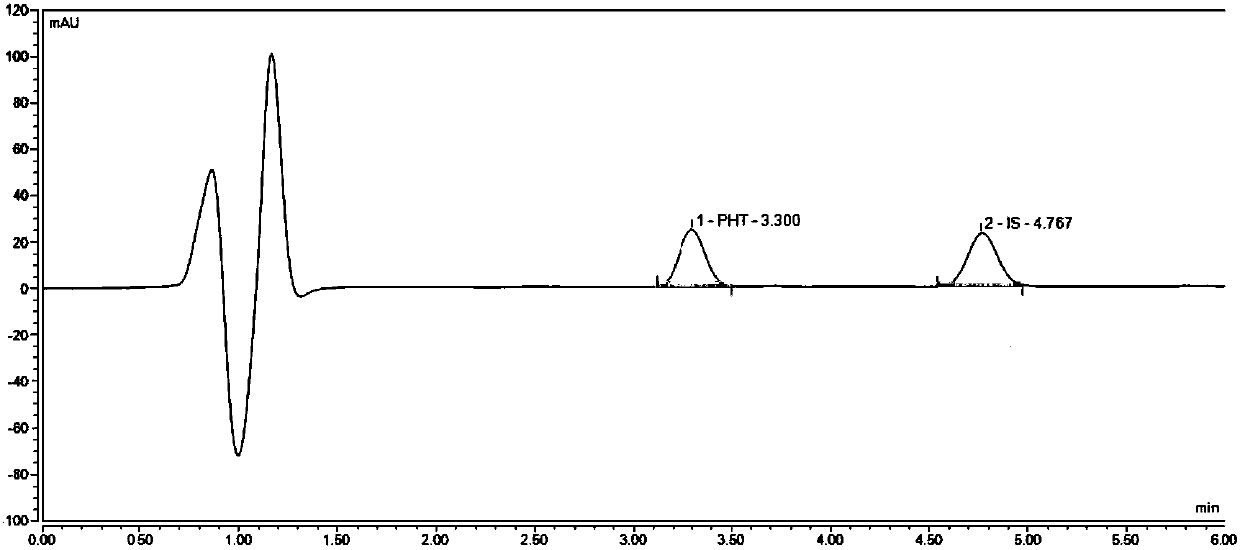
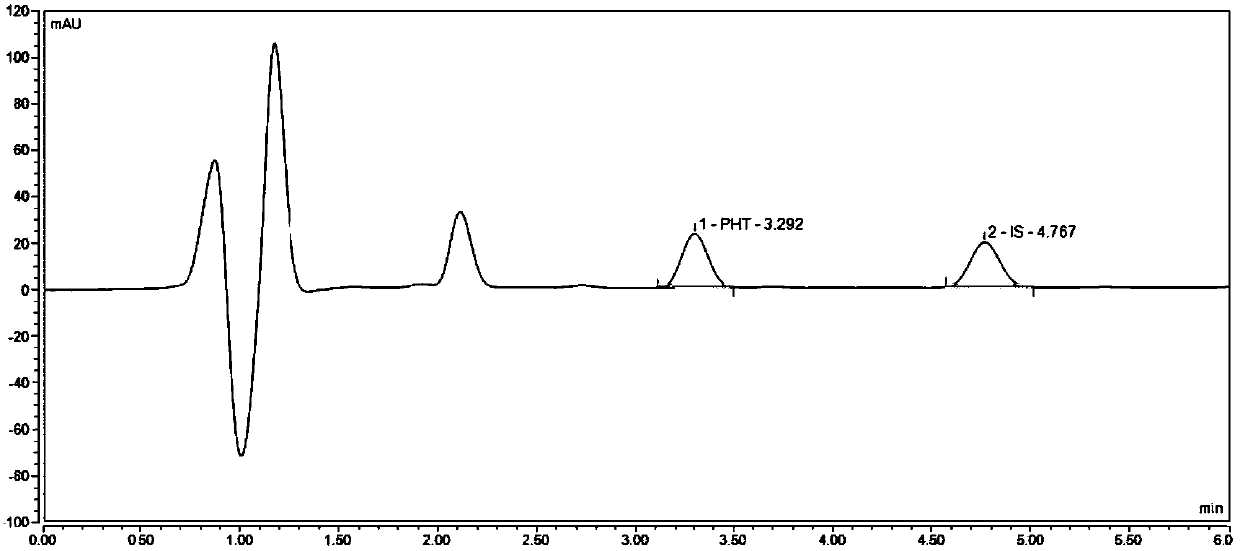
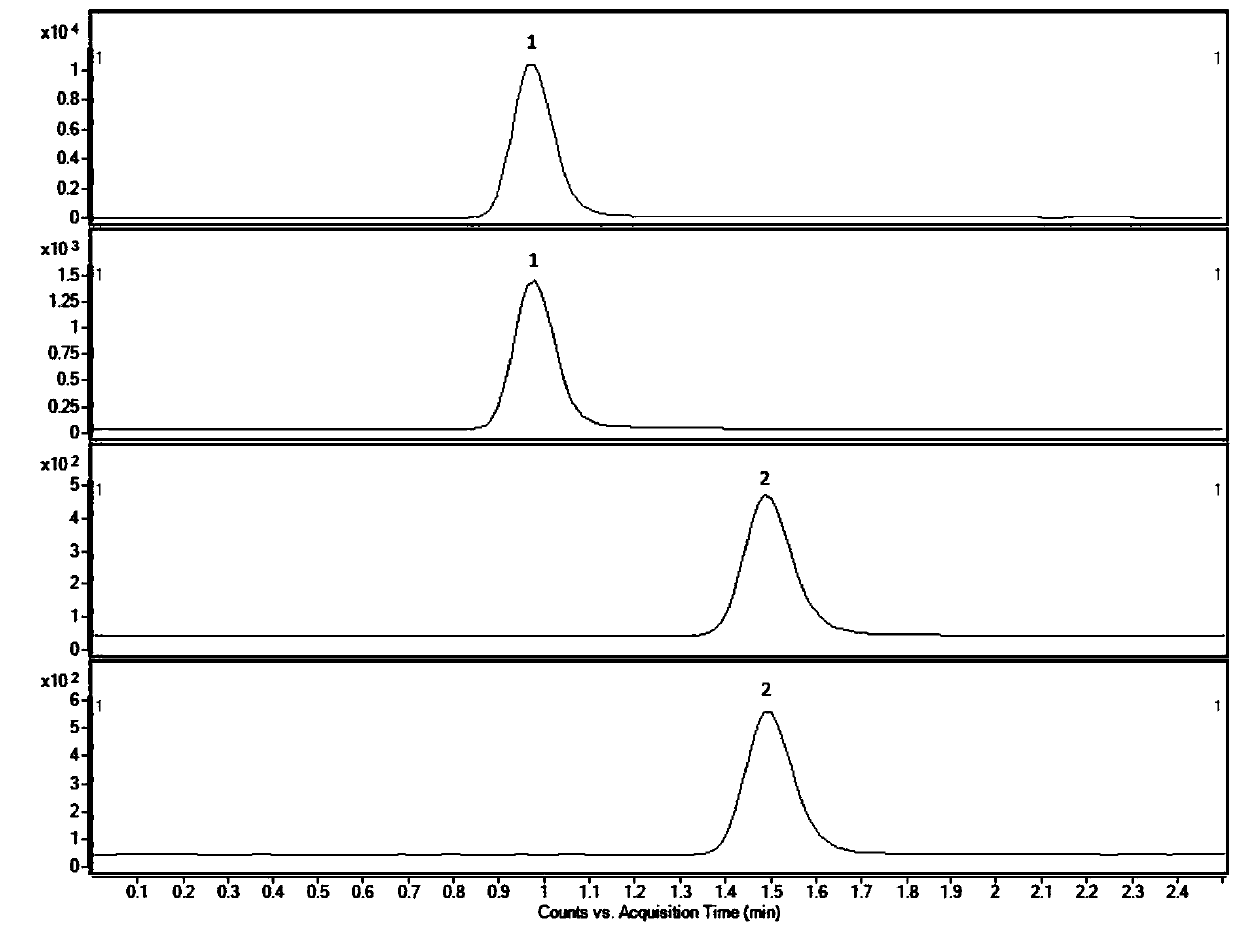
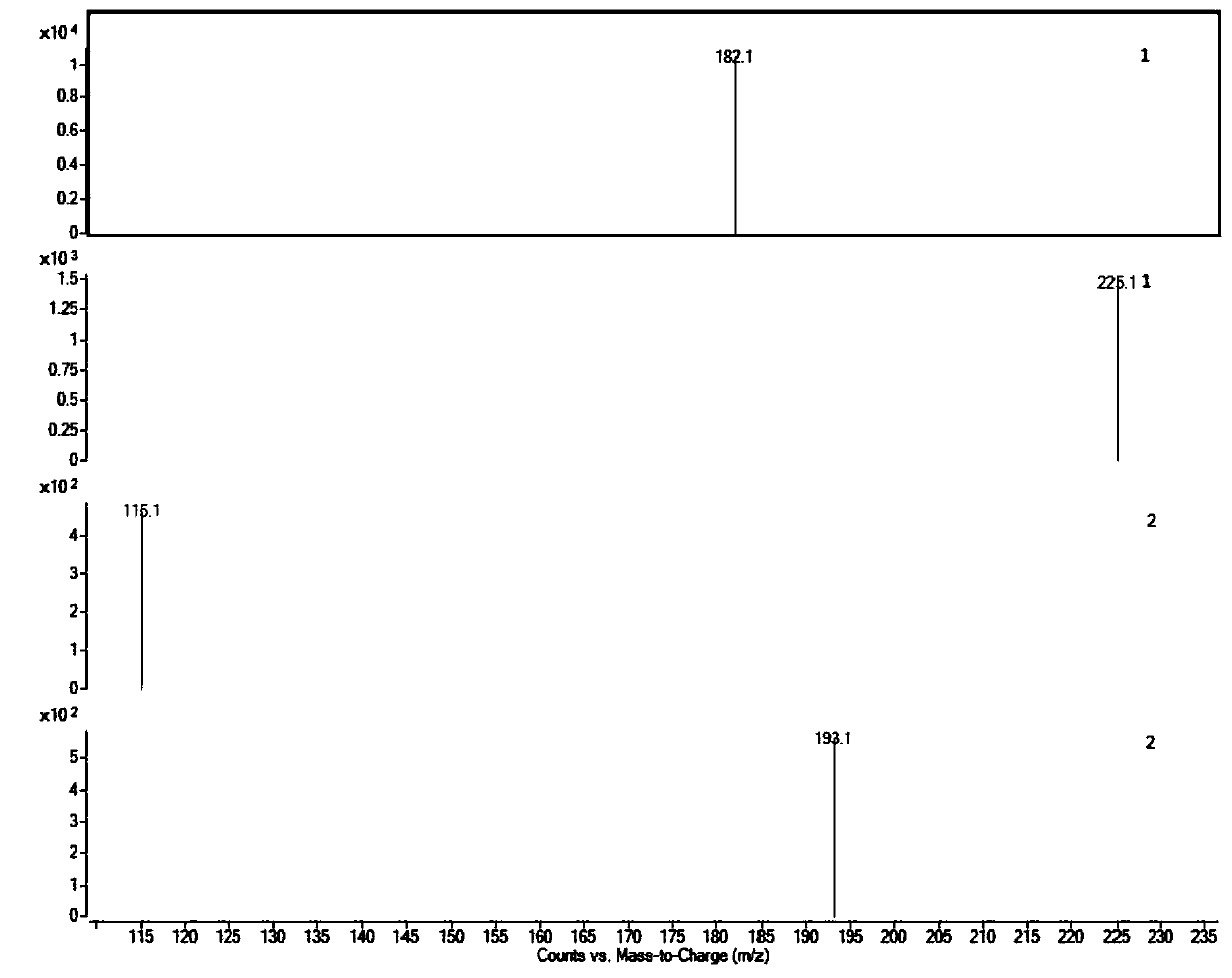
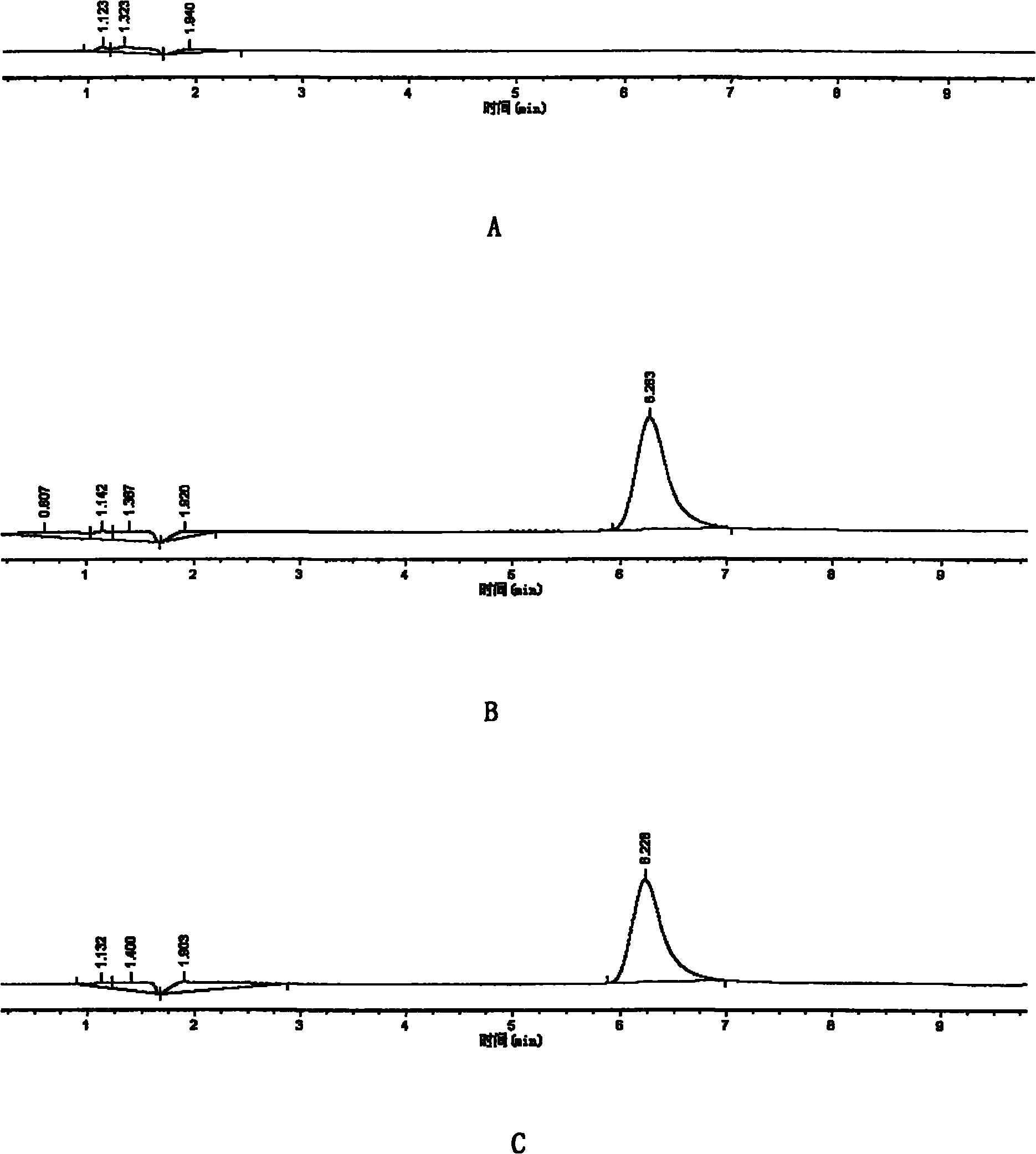
Liquid chromatogram analysis method for detecting drug content of phenytoin sodium in blood
The invention discloses a liquid chromatogram analysis method for detecting the drug content of phenytoin sodium in blood. The liquid chromatogram analysis method comprises the following steps: usinga liquid chromatogram analysis instrument and an ultraviolet detector to calibrate a standard solution, fitting to obtain a standard curve equation that y equals to the sum of a*x and b, taking a blood sample to be detected, after the blood to be detected is treated, using the liquid chromatogram analysis instrument and the ultraviolet detector to detect the sample to be detected as well to obtainy value of the blood to be detected, substituting the y of the blood to be detected into the standard curve equation, and carrying out calculation to obtain the relative concentration x of a target objective in the blood sample to be detected, wherein the concentration of the working solution of an internal standard substance is known, and the drug concentration of phenytoin sodium in the blood to be detected is calculated and obtained. According to the method, the accuracy of a quantitative result is improved, the analysis time is greatly shortened, the detection process is simple and quick,the blood concentration of phenytoin sodium inside the body of a patient during clinic treatment can be monitored better, and experimental foundation is provided for individual dosing of antiepileptic drug phenytoin sodium and the occurrence of reducing toxic and / or side effects.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Compound syrup paste for epilepsy
InactiveCN101406680ANo heredityAdjust fearHeavy metal active ingredientsOrganic active ingredientsConvulsionWarm water
The invention discloses a compound syrup paste for treating epilepsy. Ginseng, vermilion, ruddle, amber, keel, Curcuma aromatica Salisb and Sichuan troops are ground into fine power, passed through a 100 mesh sieve and added with ground phenytoin sodium tablet with even stirring to obtain compound fine powder; licorice, grass-leaved sweetflag, rhizoma pinellinae praeparata, Baikal skullcap root, cassia twig, Indian bread with hostwood and dried ginger powder are ground and passed through a 10 mesh sieve, and soaked in a 80 to 90 DEG C warm water for 2 hours twice and filtered, and the filtrates are mixed; slag is added with water, boiled for 30 minutes and filtered; the three filtrates are mixed, condensed, added with brown granulated sugar, white granulated sugar, honey and so on with even stirring and stood to obtain condensed syrup; the syrup is added with the compound powder, edible preservative, boiled to be dissolved fully, stirred evenly and stood. Combining the Chinese medical herbs and Western medicines, the compound syrup paste has functions of supplementing vigour, treating epilepsy, arresting convulsion and reducing phlegm, and promoting urination and reducing turbid pathogenic factors, overcomes the drawbacks of using single Chinese medicine and single Western medicine, and radically cure epilepsy.
Owner:王学岭
Phenytoin sodium powder injection and preparation method thereof
InactiveCN101103975AImprove solubilityHigh clarityPowder deliveryOrganic active ingredientsSide effectArginine
The invention discloses phenytoin sodium powder injection and the preparation method, relating to power injection for treating falling sickness and relating to the preparation method of the power injection. The invention is characterized in that the phenytoin sodium powder injection contains an active ingredient phenytoin and latent solvent arginine; the arginine is L-arginine or R-arginine. The phenytoin sodium powder injection in the invention, with the arginine as the latent solvent has the advantages that the dissolution is very quick; the clarity is obviously increased; no side effects are produced to patients so that the security of the injection use is increased; the lower pH than the original phenytoin sodium carbonate powder injection helps reduce thrill to the injection site and reduce pain for patients; the concentration of sodions are reduced, therefore the injection can be used to the patient who is limited to absorb sodions.
Owner:SHANGHAI NEW ASIA PHARMA
Method for rapid detection of phenytoin analogues in products
ActiveCN104215670BSimple resultIt is not easy to be unable to judge the situationMaterial electrochemical variablesUltrasonic assistedFiltration
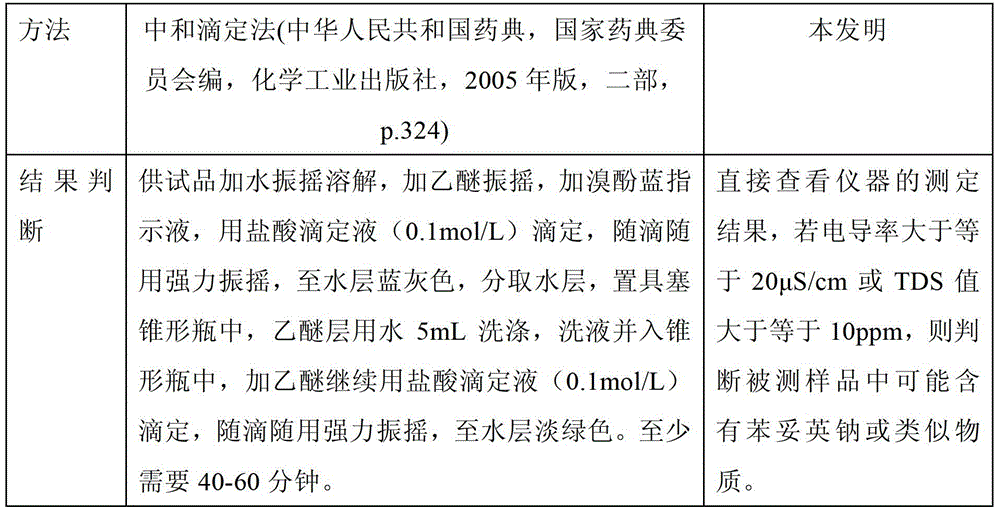
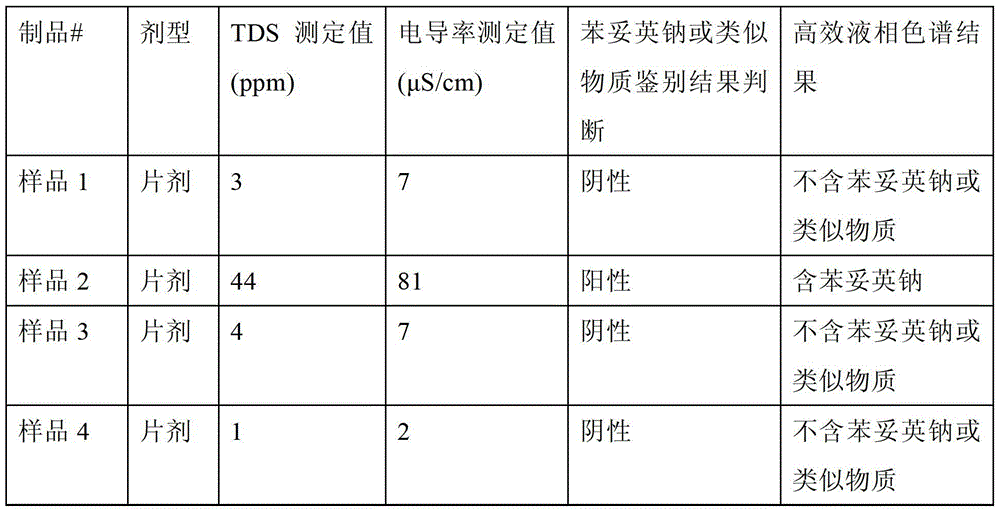
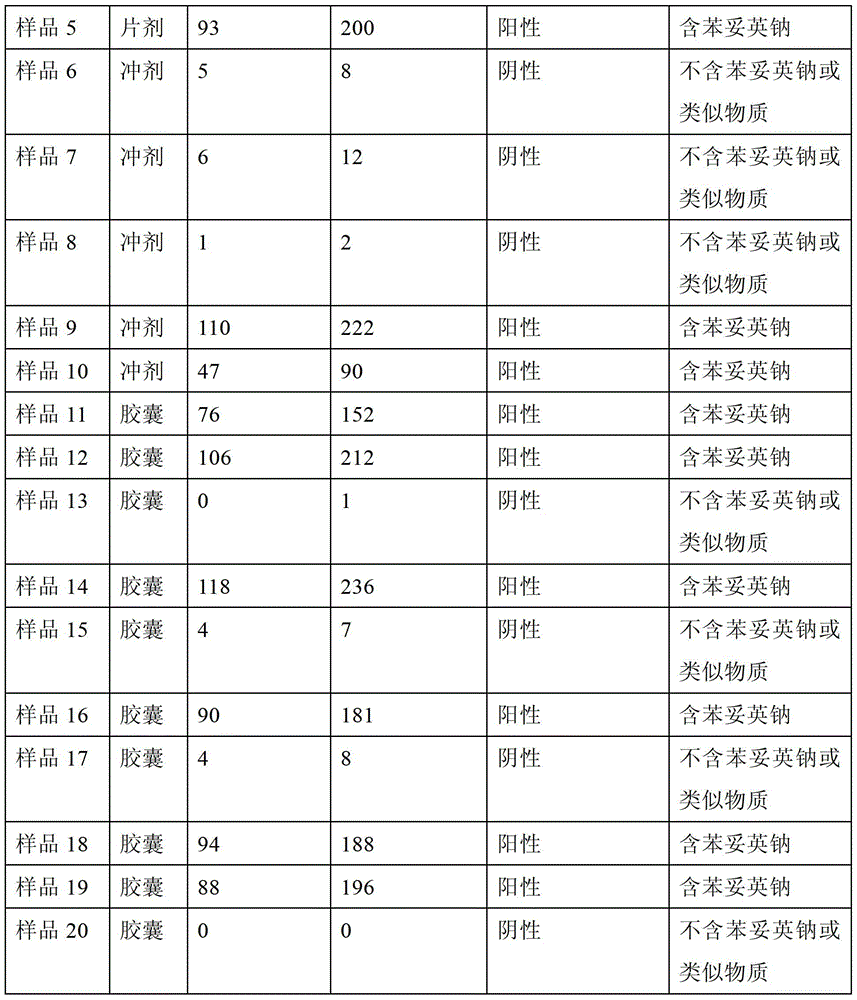
The invention relates to a method for rapidly detecting phenytoin analogues in products. It specifically relates to a method for detecting whether phenytoin sodium or similar substances are contained in products suspected of being mixed with phenytoin sodium or similar substances. (2) Use an appropriate detector based on a specific electrochemical method to measure the specific electrochemical parameters of the mixed test solution; (3) According to the value of the parameter, it can be judged whether the product may be illegally added with phenytoin Sodium or similar antiepileptic substances. The method of the invention does not need to filter the sample solution, and the whole detection process only takes 2 to 3 minutes, and has the advantages of fastness, simplicity, safety, high sensitivity, environmental protection and the like.
Owner:BEIJING INST FOR DRUG CONTROL
Medicinal composition for treating pulpitis
InactiveCN105616425AEnhance anti-inflammatoryEnhanced AntihistamineDigestive systemHeterocyclic compound active ingredientsAnaerobic bacteriaMigraine
Owner:匡永刚
Method for detecting phenytoin sodium in blood
PendingCN110632235AShorten detection timeAccurate identificationComponent separationInternal standardSystem error
A method for detecting phenytoin sodium in blood comprises the steps of: preparing a standard stock solution of phenytoin sodium, preparing a standard intermediate solution of phenytoin sodium, preparing an internal standard working solution, preparing a standard solution, detecting the standard solution by using a liquid chromatography-mass spectrometer, and fitting to obtain a standard curve equation corresponding to the phenytoin sodium, wherein the standard curve equation is as follows: y<1>=a*x<1>+b; processing blood to be detected, detecting the blood to be detected by using the liquid chromatogram-mass spectrometer, and calculating phenytoin sodium concentration in the blood to be detected. According to the method of the invention, the processing method of a blood sample to be detected and the selection of an internal standard substance enable the identification of the phenytoin sodium to be accurate, the analysis time is short, the interference is small, the internal standard quantification is suitable, the specificity is strong, the sensitivity is high, and meanwhile, all technical indexes such as a recovery rate, a detection limit and a precision meet the requirements, sothat the accuracy of a detection result is improved, and a system error is eliminated.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
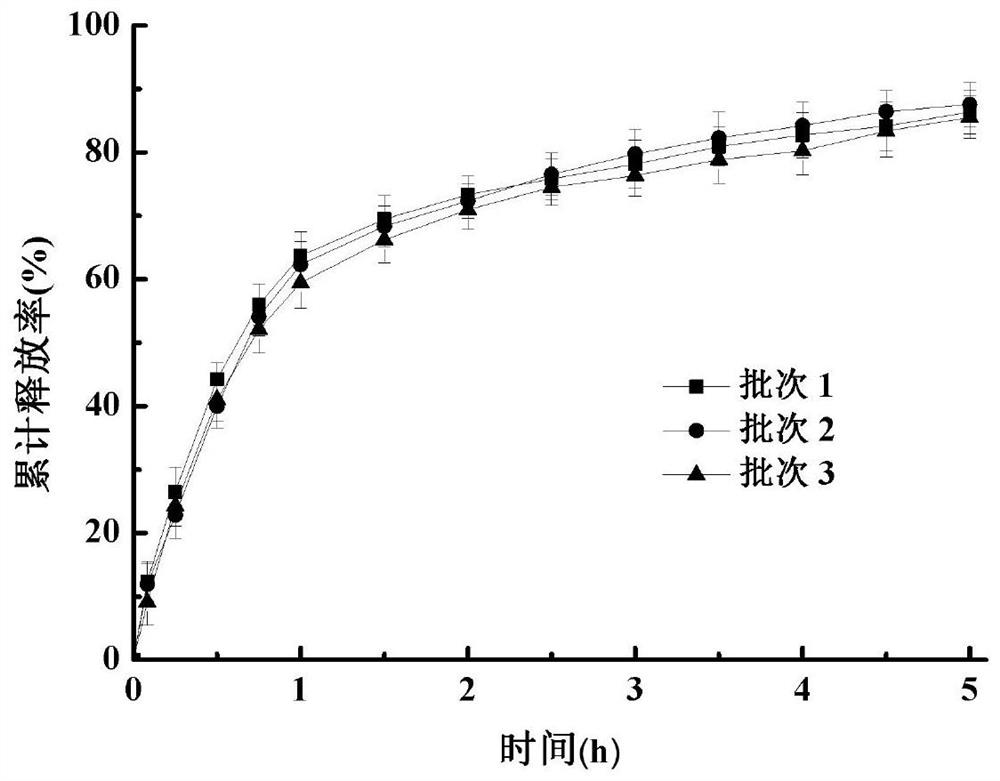
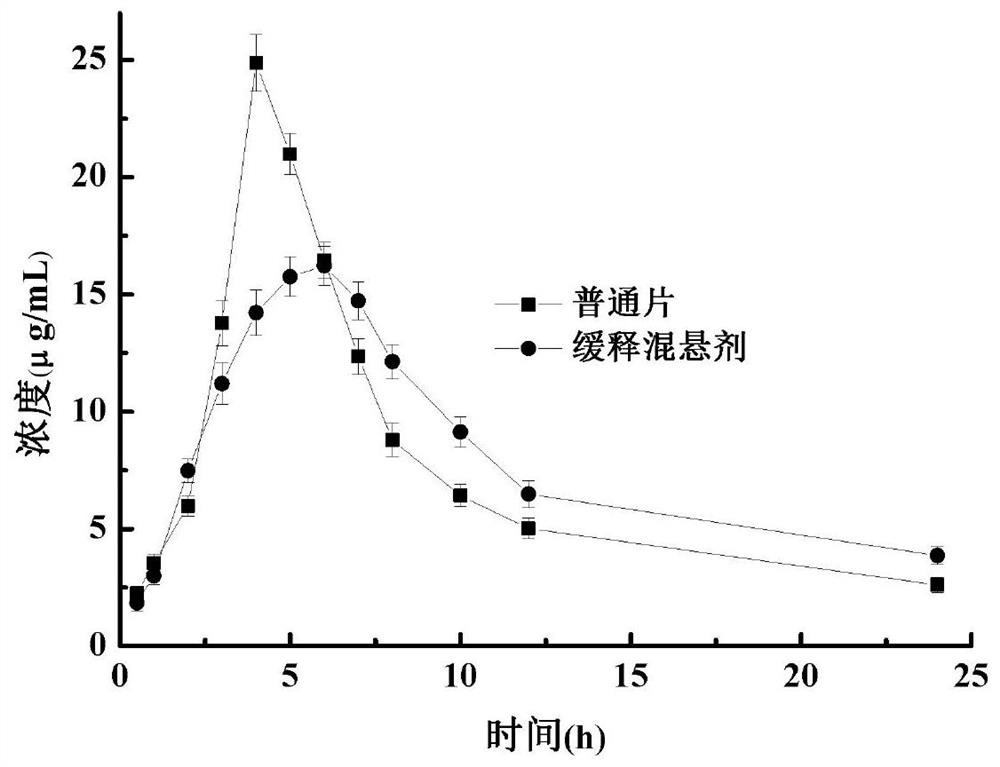
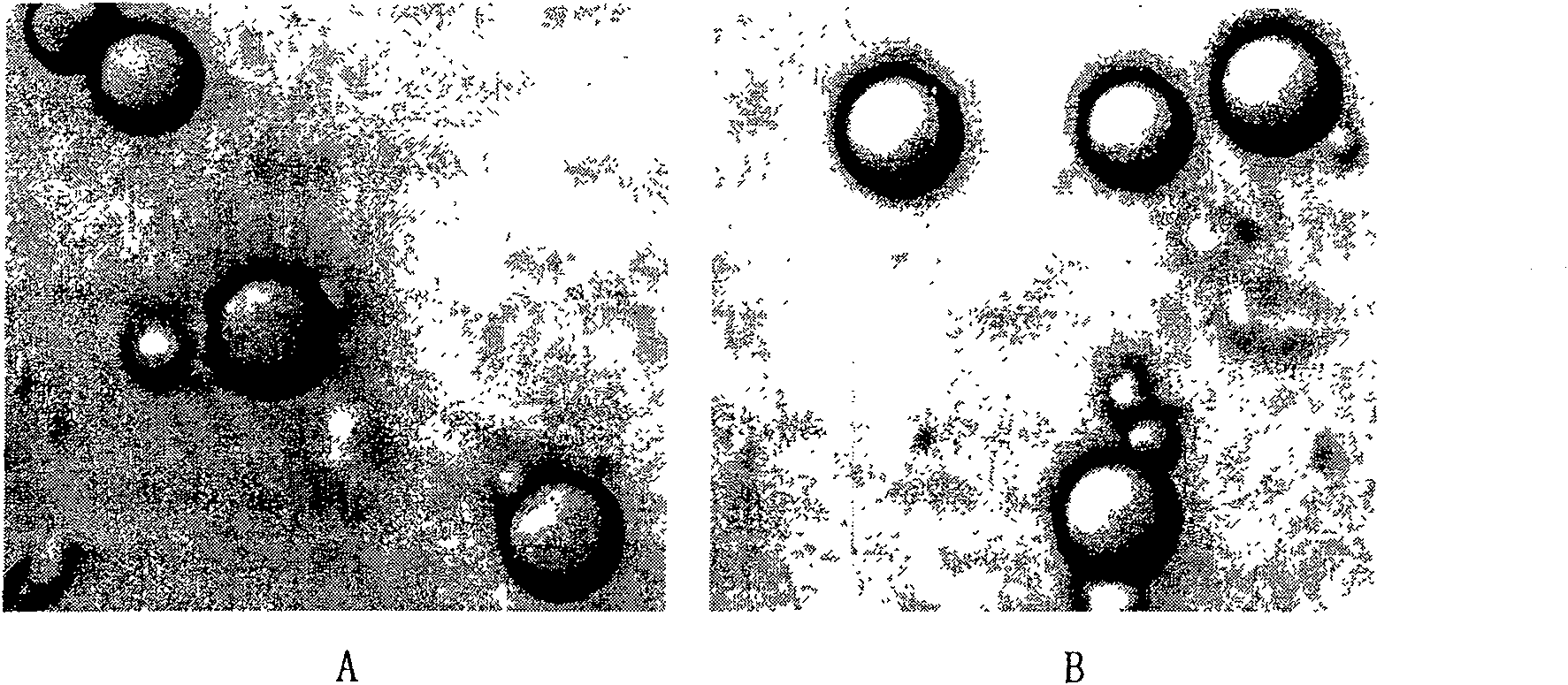
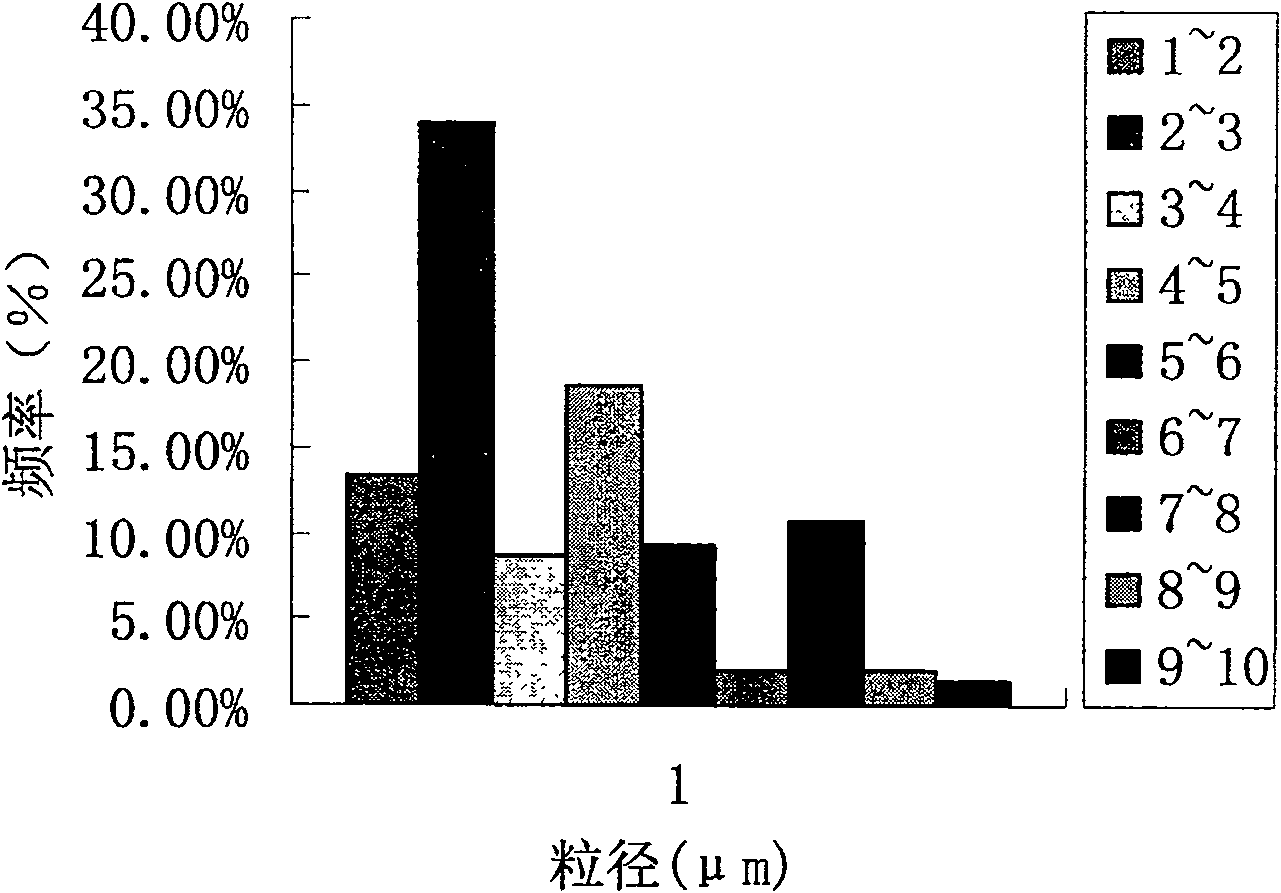
Phenytoin sodium sustained-release suspension and preparation method thereof
InactiveCN111686074AImprove stabilityStable drug release in vivoOrganic active ingredientsNervous disorderPlasticizerSuspending Agents
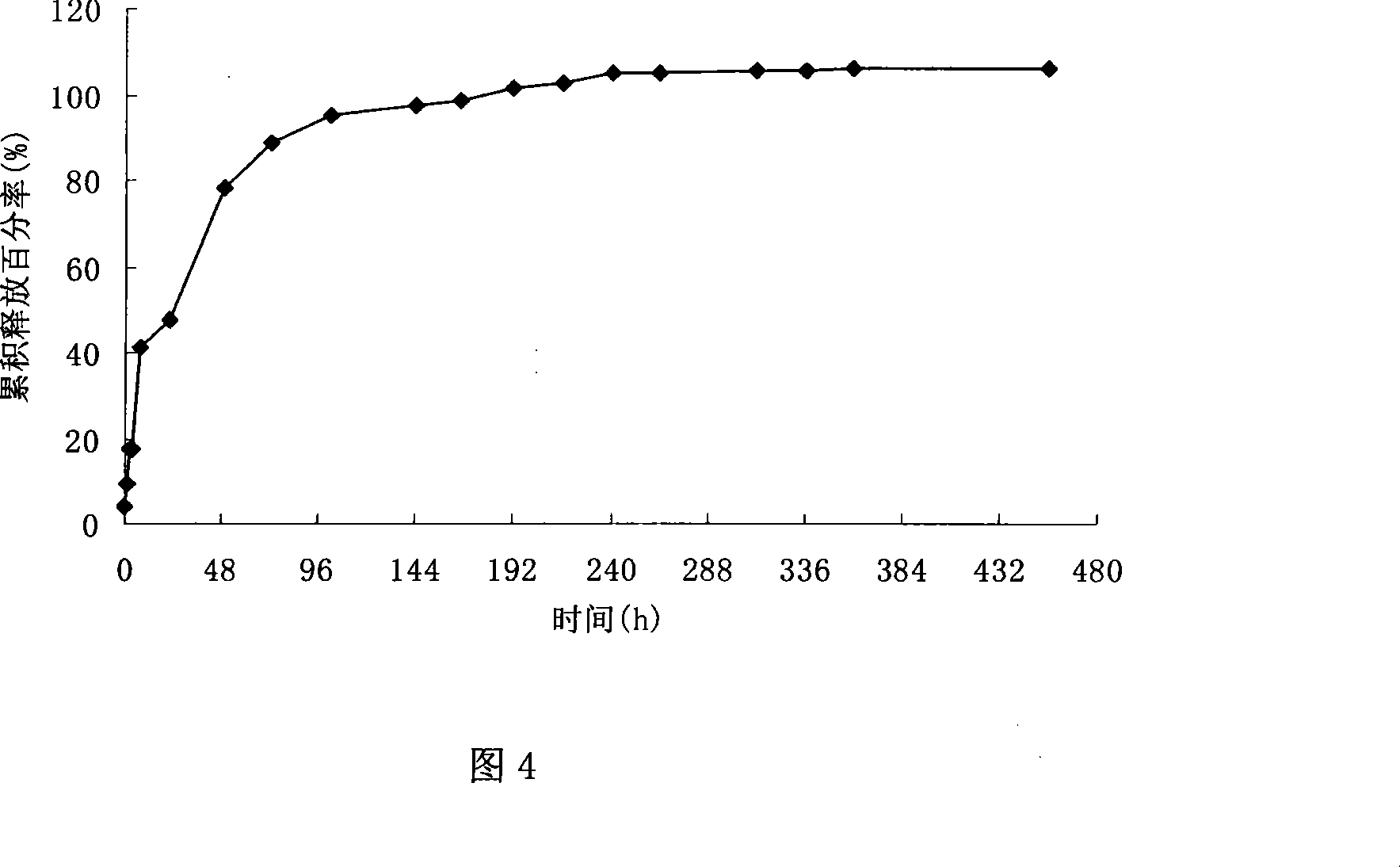
The present invention discloses a phenytoin sodium sustained-release suspension and a preparation method thereof, and belongs to the technical field of sustained-release preparations. The phenytoin sodium sustained-release suspension comprises phenytoin sodium, sustained-release auxiliary materials, impregnating agents, plasticizers, suspending agents, and wetting agents; and mass ratios of the phenytoin sodium, sustained-release auxiliary materials, impregnating agents, plasticizers and suspending agents are 10:(10-60):(0.1-10):(0.01-2):(0.1-2). The provided phenytoin sodium sustained-releasesuspension has good stability, an in vitro release rate of 75%-85% in 5 hours, a stable in vivo drug release, a long duration of drug effects and broad prospects in clinical applications.
Owner:JIANGSU SIHUAN BIOENGINEERING PHARM CO LTD
Medicine composition for treating abdominal angina syndrome
InactiveCN105477057APromote recoveryImprove immunityHeavy metal active ingredientsAntipyreticBlood vesselPhenytoin Sodium
The invention discloses a medicine composition for treating abdominal angina syndrome. The composition is prepared from the following components: coronarine, olein, dilantin, total flavonoids of perilla leaf, paeoniflorin, ethanol, vitamin B1, sodium carboxymethyl starch, cisapride, ferrous succinate, buprenorphine, a filling agent, a stabilizing agent, a preservative and an antioxidant. The medicine composition has various efficacies of stopping pain, resisting bacteria, expanding blood vessel, resisting coagulation, promoting digestive system recovery, and preventing intestinal obstruction, vascular malformation, anemia and other complications, and is remarkable in curative effect of abdominal angina syndrome treatment.
Owner:孙晓英
Powdered medicine for treating intractable ulcer
InactiveCN1199666CEasy to synthesizeSpeed up healingPowder deliveryInorganic boron active ingredientsVitamin CCure rate
A medicine "Suyusan" for treating intractable ulcer is prepared from phenytoin sodium, sodium benzoate, VC and 5 Chinese medicinal materials including pearl layer powder, borax, borneol, etc. throughuniformly mixing according to defined quantity. Its advantages include speeding up healing, no any toxic by-effect, high cure rate up to 98.7%, and no recurrence.
Owner:张美琴
Compound syrup paste for epilepsy
The invention discloses a compound syrup paste for treating epilepsy. Ginseng, vermilion, ruddle, amber, keel, Curcuma aromatica Salisb and Sichuan troops are ground into fine power, passed through a 100 mesh sieve and added with ground phenytoin sodium tablet with even stirring to obtain compound fine powder; licorice, grass-leaved sweetflag, rhizoma pinellinae praeparata, Baikal skullcap root, cassia twig, Indian bread with hostwood and dried ginger powder are ground and passed through a 10 mesh sieve, and soaked in a 80 to 90 DEG C warm water for 2 hours twice and filtered, and the filtrates are mixed; slag is added with water, boiled for 30 minutes and filtered; the three filtrates are mixed, condensed, added with brown granulated sugar, white granulated sugar, honey and so on with even stirring and stood to obtain condensed syrup; the syrup is added with the compound powder, edible preservative, boiled to be dissolved fully, stirred evenly and stood. Combining the Chinese medical herbs and Western medicines, the compound syrup paste has functions of supplementing vigour, treating epilepsy, arresting convulsion and reducing phlegm, and promoting urination and reducing turbid pathogenic factors, overcomes the drawbacks of using single Chinese medicine and single Western medicine, and radically cure epilepsy.
Owner:王学岭
An alepsin slow-releasing gel for promoting paradontal part reborn as well as preparation method and application
InactiveCN101156849BSave human effortSave moneyOrganic active ingredientsDigestive systemSustained release drugMicrosphere
The invention discloses phenytoin sodium sustained-release gel which promotes periodontal regeneration, and the phenytoin sodium sustained-release gel is made of components of phenytoin sodium, PLGA sustained-release microspheres and poloxamer gel, wherein, the content of an active component, the phenytoin sodium is 2 to 5 mg / ml. The preparation method of the phenytoin sodium sustained-release gel which promotes the periodontal regeneration has the steps that firstly, the PLGA microspheres containing the phenytoin sodium is prepared in W / O / W type emulsified solvent volatilization method; secondly, the microspheres are mixed into 25 percent of the poloxamer gel, to produce microsphere gel containing 2 to 5 mg / ml phenytoin sodium. The invention provides a new safe, effective drug with acceptable price and easy promotion and application for local administration of periodontal disease therapy. The preparation method of the phenytoin sodium sustained-release gel provided by the invention is convenient, and has strong operability, the prepared sustained-release drug has stable performance, and the distribution of the microsphere graininess is more uniform.
Owner:SHANDONG UNIV
Phenytoin sodium dropping pill and preparation method thereof
InactiveCN103479591AEasy to useImprove Medication AdherenceOrganic active ingredientsNervous disorderControl disordersTherapeutic effect
The invention belongs to the technical field of medicines, and relates to a phenytoin sodium dropping pill and a preparation method thereof. The phenytoin sodium dropping pill is applicable to treatment of generalized tonic-clonic seizures, complex partial seizures (psychomotor seizures and temporal lobe epilepsy) simple partial seizures (focal seizures) and status epilepticus, can also be used for treating trigeminal neuralgia, epidermolysis bullosa dystrophica recessive, paroxysmal choreoathetosis, paroxysmal control disorders (including behavioral disorders caused by hyperexcitability such as rage, anxiety, insomnia and the like), myotonia, cardiac conduction disorder during overdose of a tricyclic antidepressant and the like, and is also applicable to ventricular and supraventricular arrhythmias caused by digitalis toxication, with a poor therapeutic effect on cardiac arrhythmias caused by other various causes. Compared with currently marketed tablet products, the phenytoin sodium dropping pill has the characteristics of simple and feasible preparation process, rapid absorption and high bioavailability.
Owner:天津市聚星康华医药科技有限公司
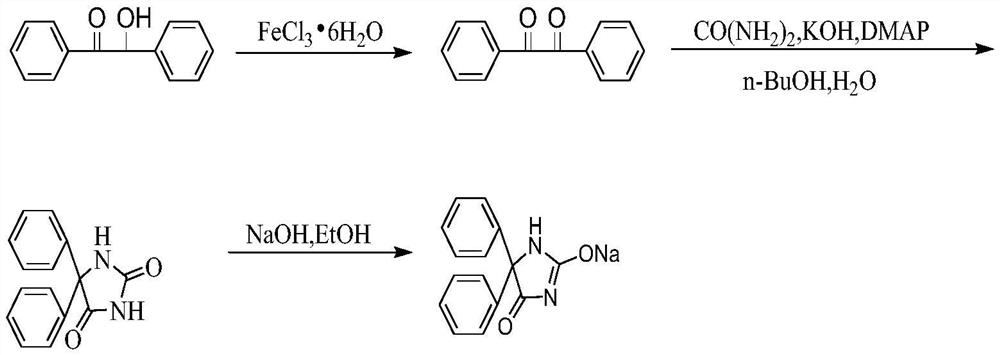
A kind of synthetic method of sodium phenytoin
ActiveCN109456271BReduce the reaction concentrationSuppress generationOrganic chemistryDiphenylacetyleneBiomedicine
The invention relates to a method for synthesizing phenytoin sodium, which belongs to the technical field of biomedicine. The synthetic method of phenytoin sodium of the present invention comprises oxidation reaction, condensation reaction, salt-forming reaction, in condensation reaction, has promoted amidation reaction process by adding phase-transfer catalyst 4-dimethylaminopyridine; Adopt n-butanol and water two-phase system , greatly inhibited the generation of by-product diphenylacetylene diurea, and the reaction time was significantly shortened; in the salt-forming reaction, ethanol was used as a solvent, and after the reaction of sodium hydroxide ethanol solution and phenytoin, cyclohexane, a poor solvent, was added to promote the reaction of phenytoin Sodium is precipitated as white crystals. Compared with the salt-forming reaction in which water is used as a solvent, phenytoin sodium precipitates at a faster rate, with a higher degree of precipitation and higher purity.
Owner:NINGBO POLYTECHNIC
Efficient detergent for fabrics
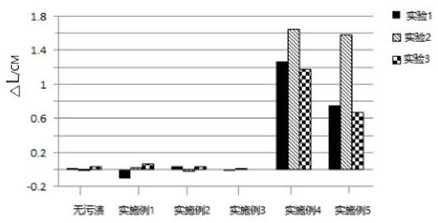
PendingCN113234547AProcess cleaningWon't wear outInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsPerylene derivativesStain remover
The invention provides an efficient detergent for fabrics. The efficient detergent comprises an oil stain detergent and a solid stain detergent, and the oil stain detergent and the solid stain detergent both comprise phenytoin sodium derivatives. The detergent disclosed by the invention has the beneficial effects that the detergent disclosed by the invention is pure neutral, does not hurt fabrics and skins, has a very good decontamination effect on intractable oil stains and solid stains, and also has a very obvious effect on stains which are dried and hardened on the surfaces of the fabrics for a long time.
Owner:德州竹洋洗涤科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com