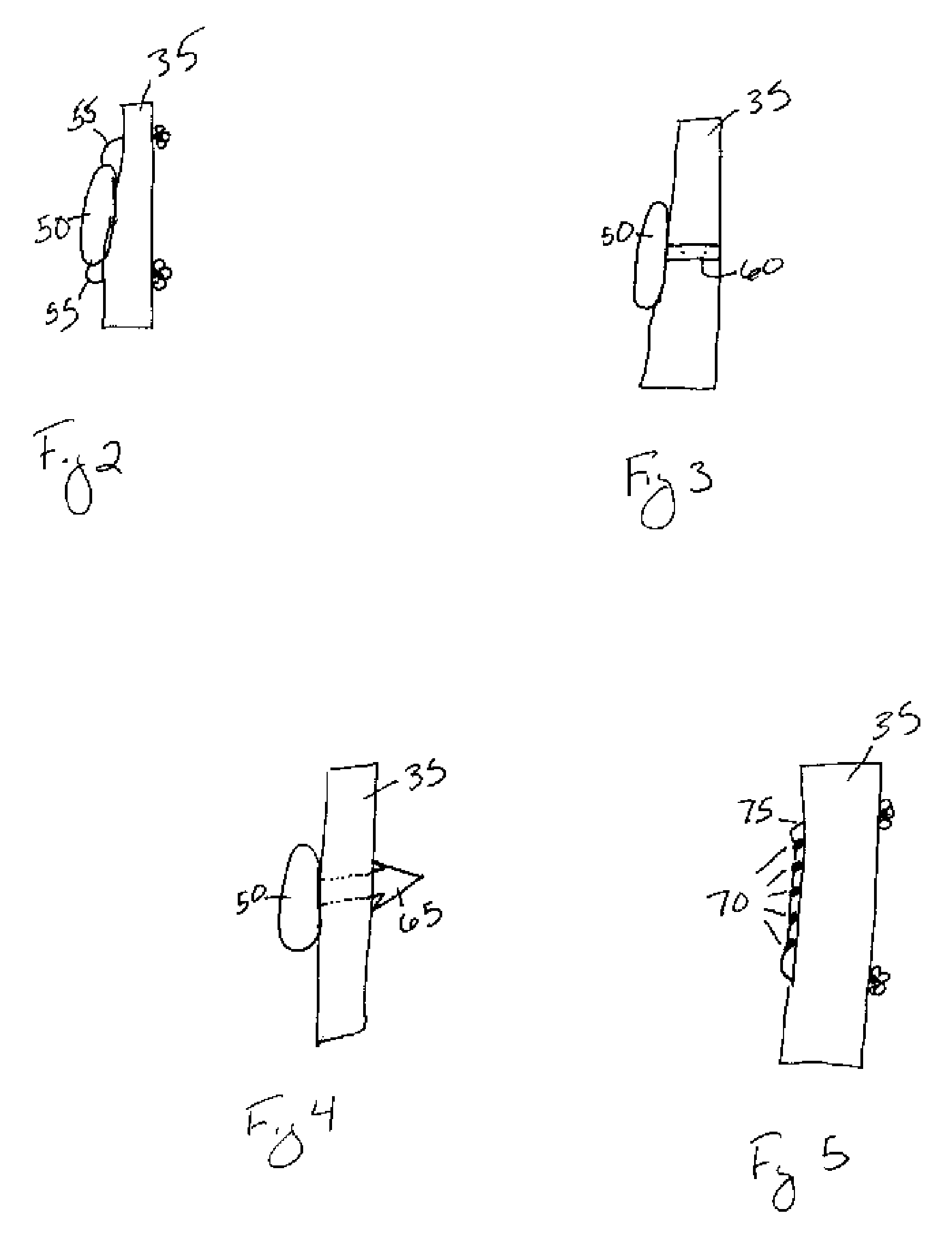Patents
Literature
283 results about "Enteral administration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enteral administration is food or drug administration via the human gastrointestinal tract. This contrasts with parenteral nutrition or drug administration (Greek para, "besides" + enteros), which occurs from routes outside the GI tract, such as intravenous routes. Enteral administration involves the esophagus, stomach, and small and large intestines (i.e., the gastrointestinal tract). Methods of administration include oral, sublingual (dissolving the drug under the tongue), and rectal. Parenteral administration is via a peripheral or central vein. In pharmacology, the route of drug administration is important because it affects drug metabolism, drug clearance, and thus dosage. The term is from Greek enteros, "intestine".
Nutritional supplements
InactiveUS20060088574A1Pleasant tasteEncouraging consumption of nutritional supplementVitamin food ingredientsPharmaceutical non-active ingredientsNormal blood glucoseNutrition
The invention provides nutritional supplements for an oral or enteral administration to humans, whether in satisfactory health, or having a medical condition. These supplements can provide humans with a complete, balanced nutrition in a bioavailable form, either as a food supplement or as a meal replacement, and preferably includes one or more slow-digesting carbohydrates that renders them effective in maintaining blood glucose levels at, or returning abnormal blood glucose levels to, normal blood glucose levels. Nutritional supplements within the invention can be employed with humans that are diabetic, borderline diabetic, pregnant and / or lactating, geriatric humans and humans that have, are at risk for, or develop other glucose intolerance or cardiovascular disease.
Owner:PBM PRODUCTS INC
Rapid acting drug delivery compositions
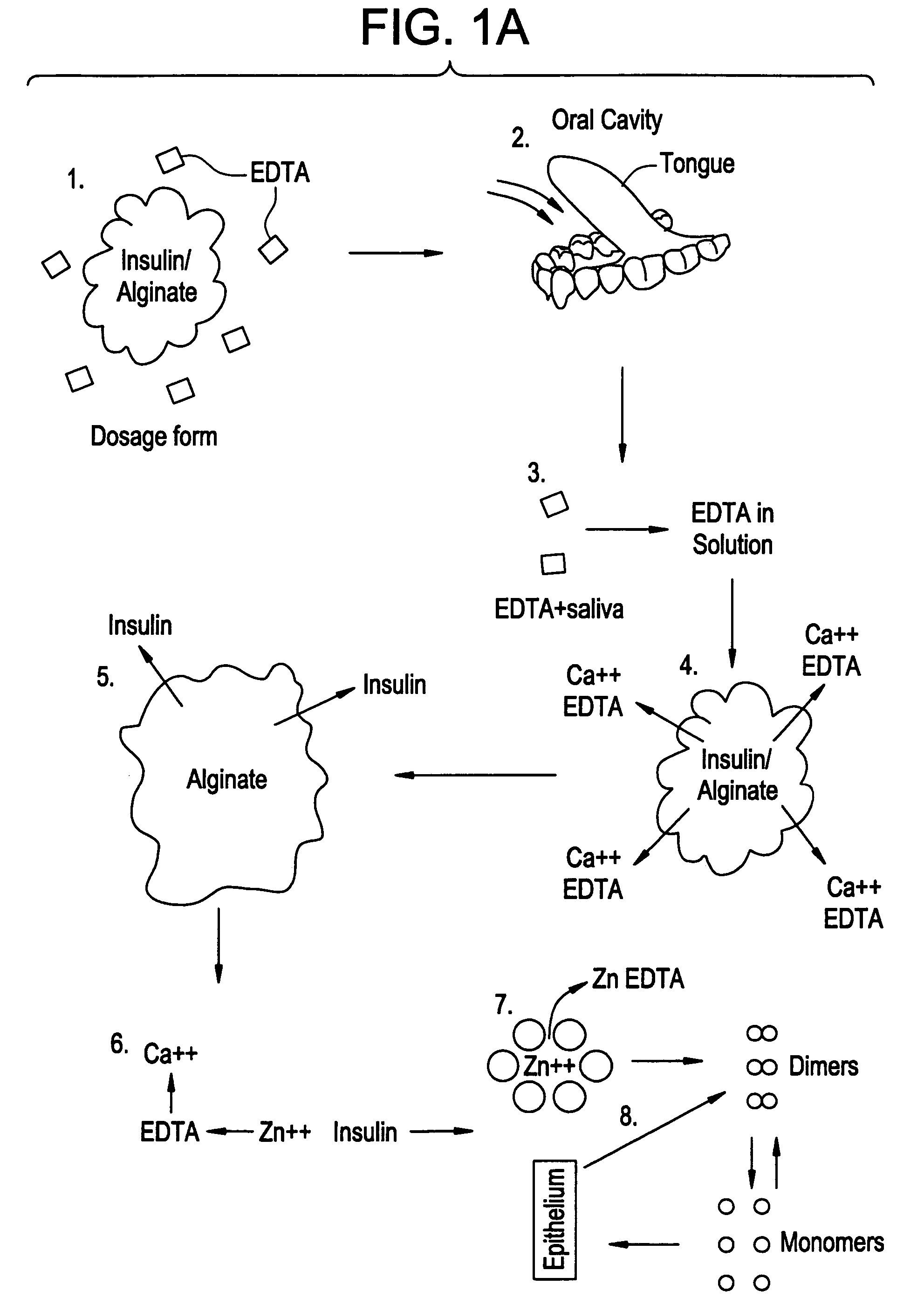
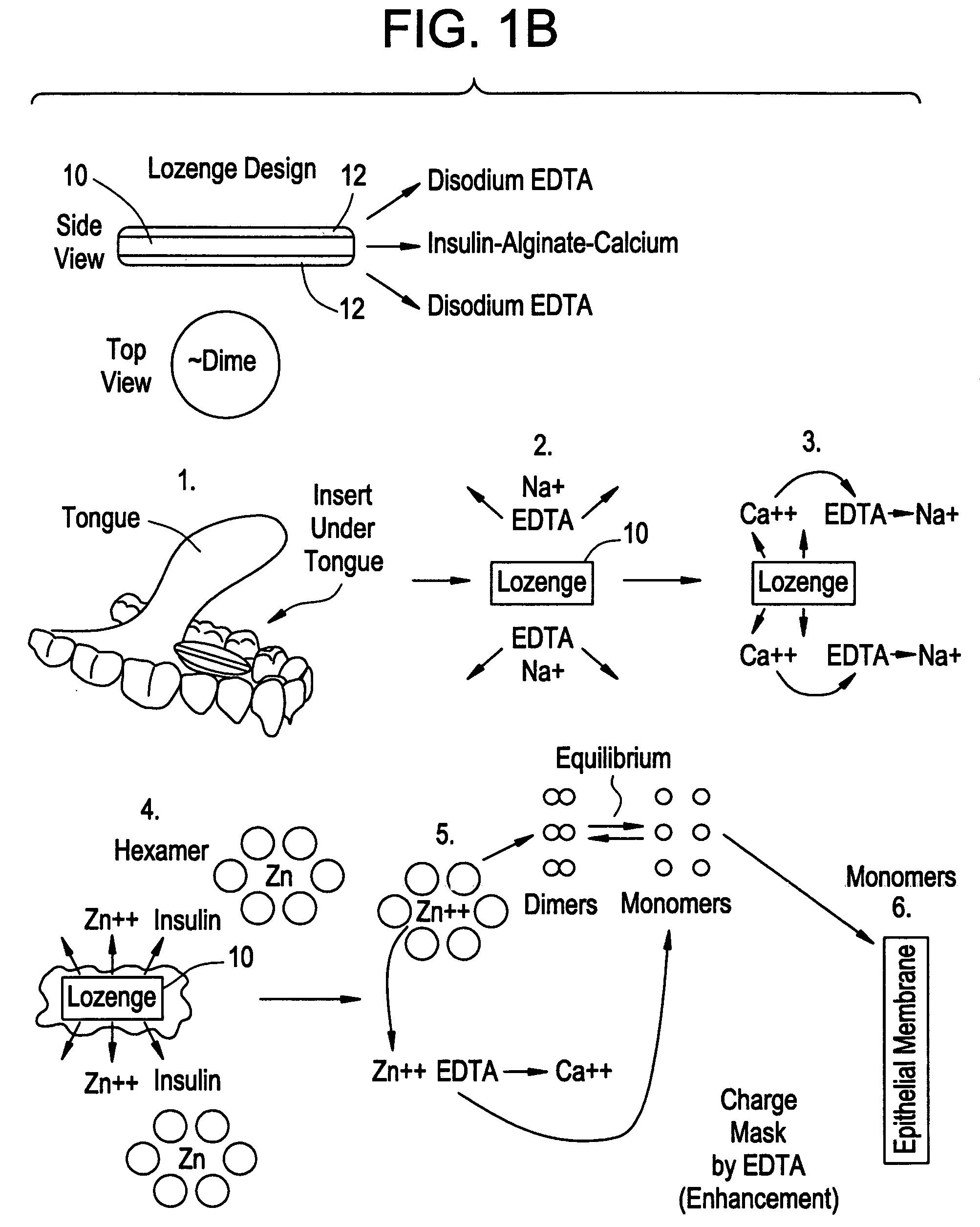
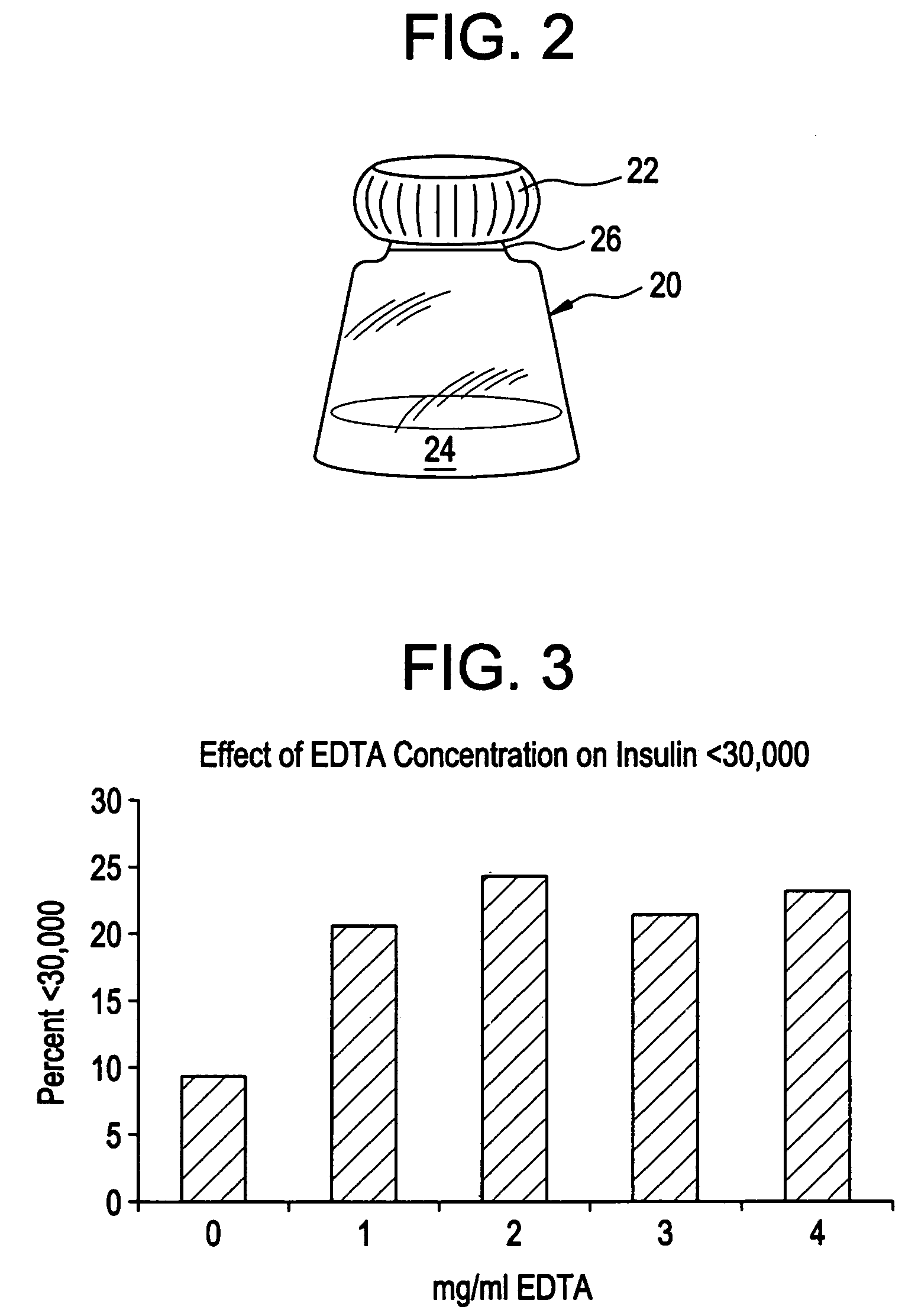
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
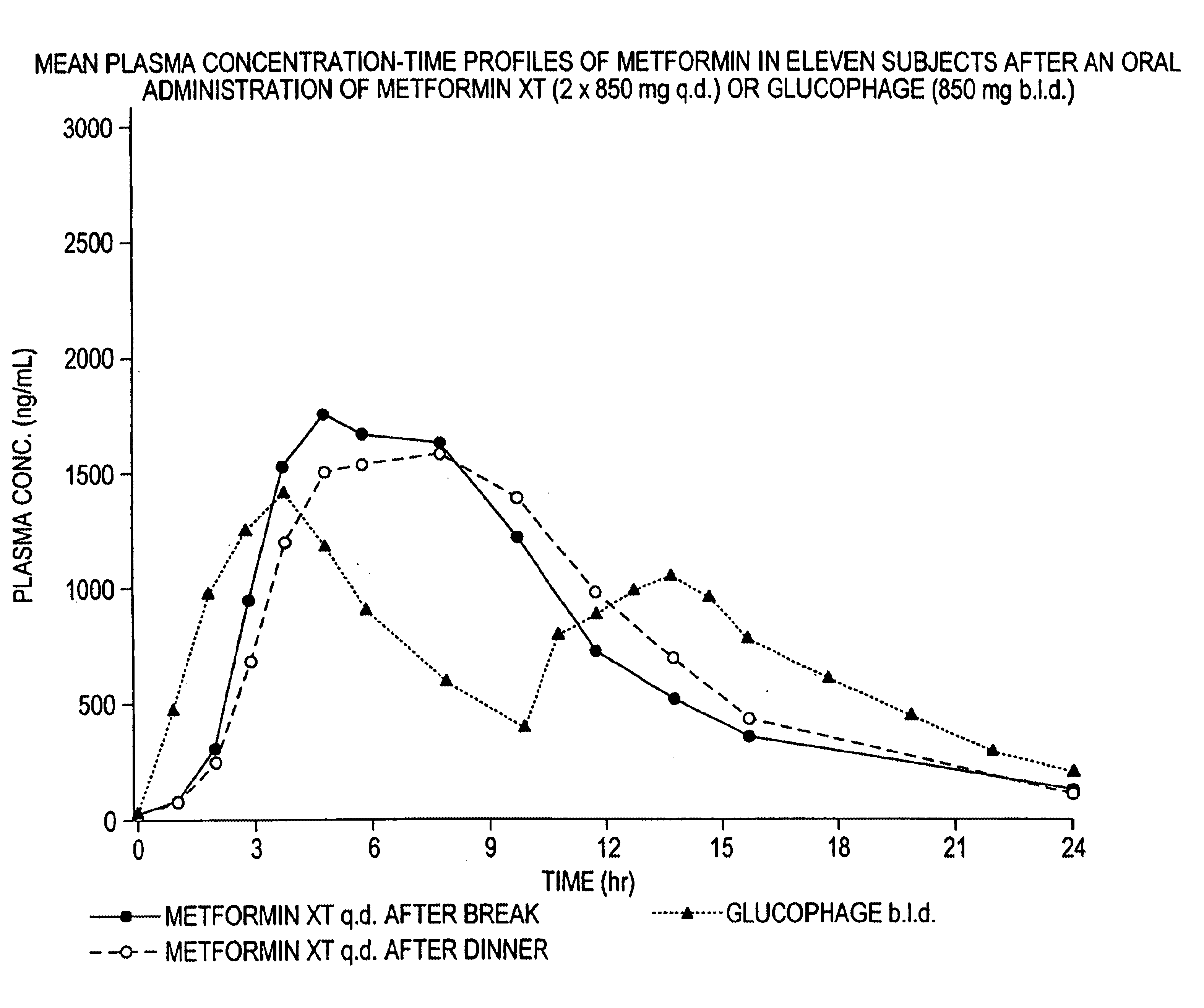
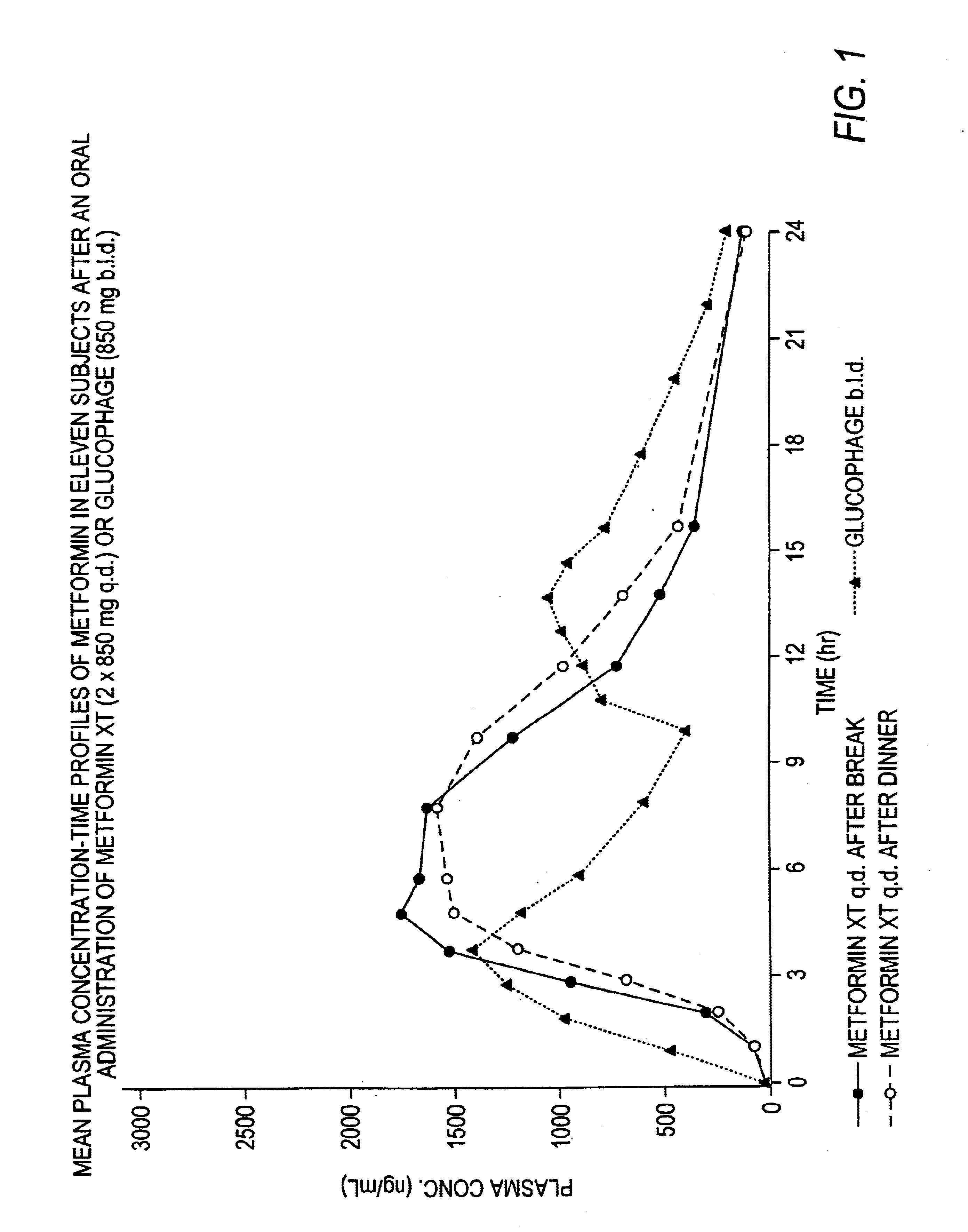
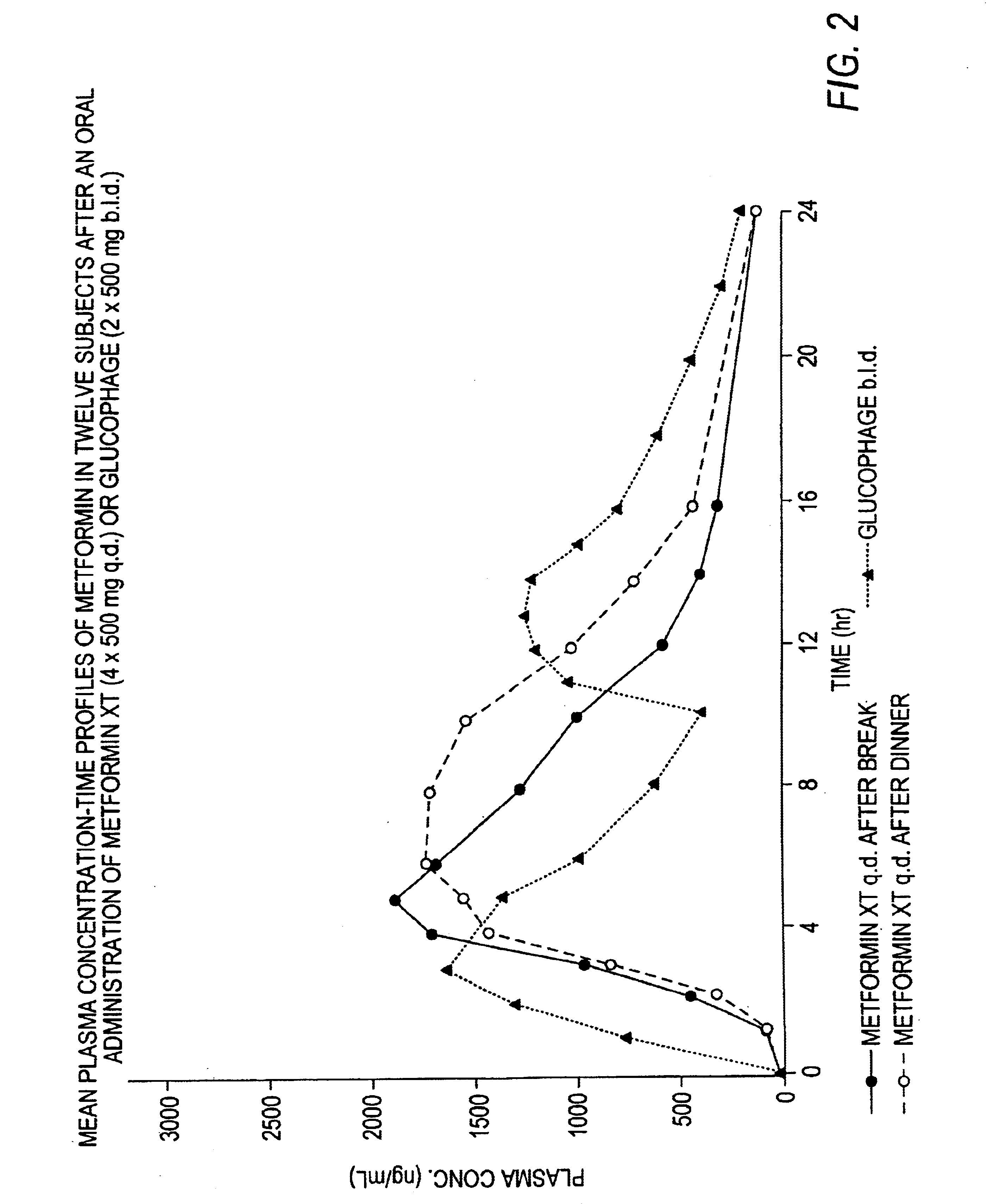
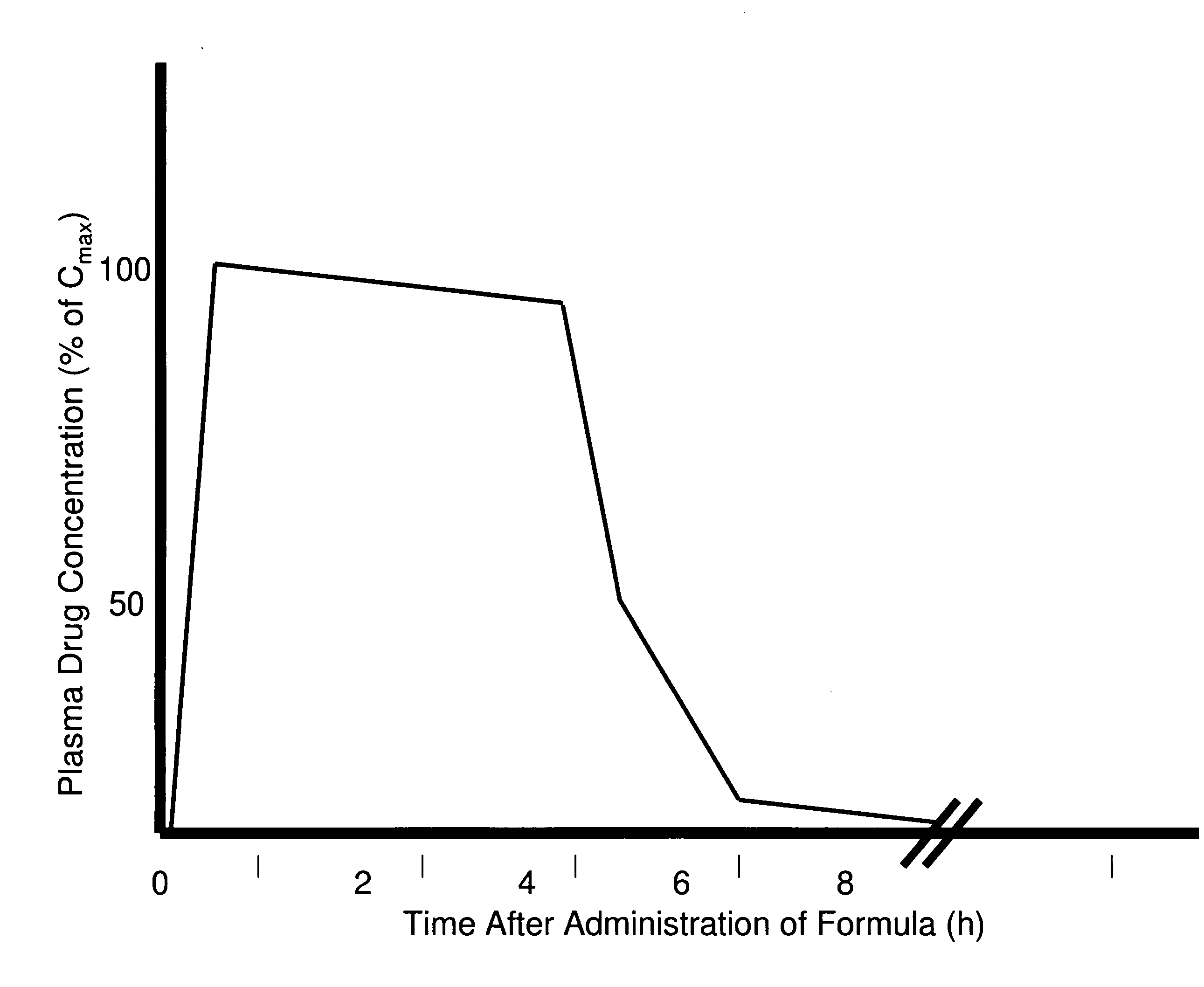
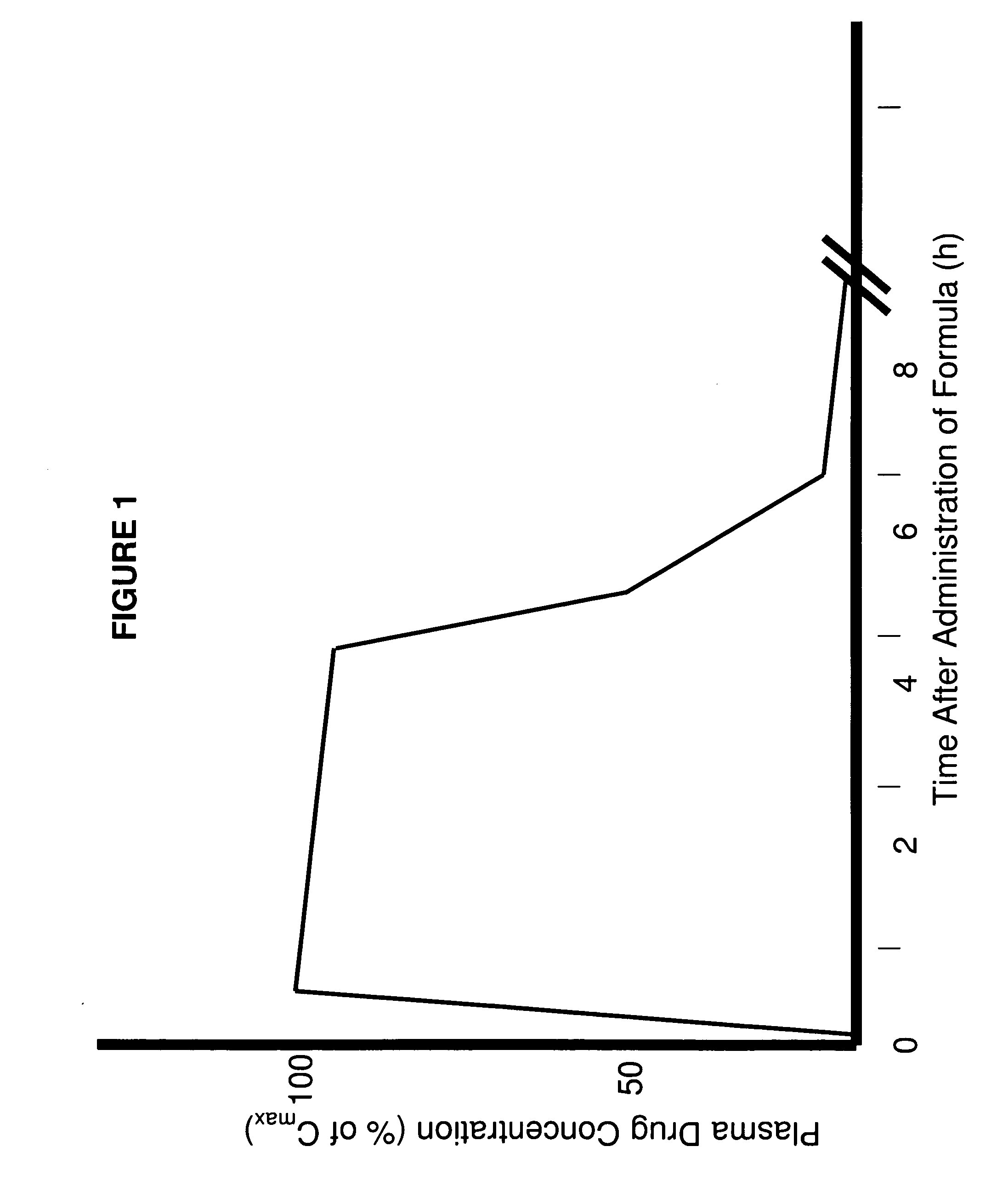
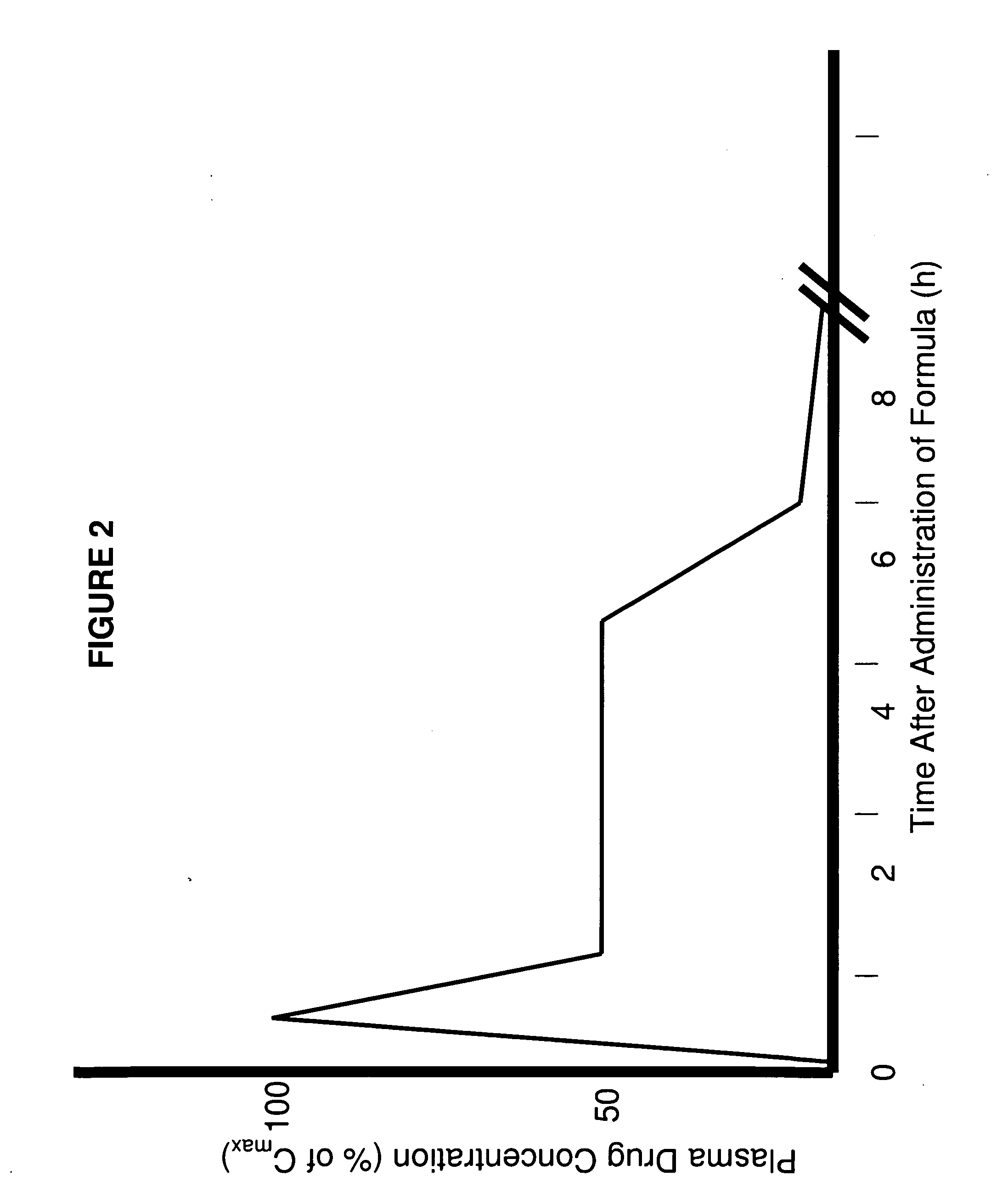
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Parenteral Formulations of Dopamine Agonists
ActiveUS20090143390A1Reducing elevated cardiovascular-related inflammatory factorReducing elevated cardiovascular-related inflammatory factorsBiocideAntipyreticEnteral administrationParenteral Dosage Form
This invention relates to stable pharmaceutical compositions for parenteral administration comprising dopamine agonists and peripheral acting agents_useful for treatment of metabolic disorders or key elements thereof. The parenteral dosage forms exhibit long stable shelf life and distinct pharmacokinetics.
Owner:VEROSCI
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
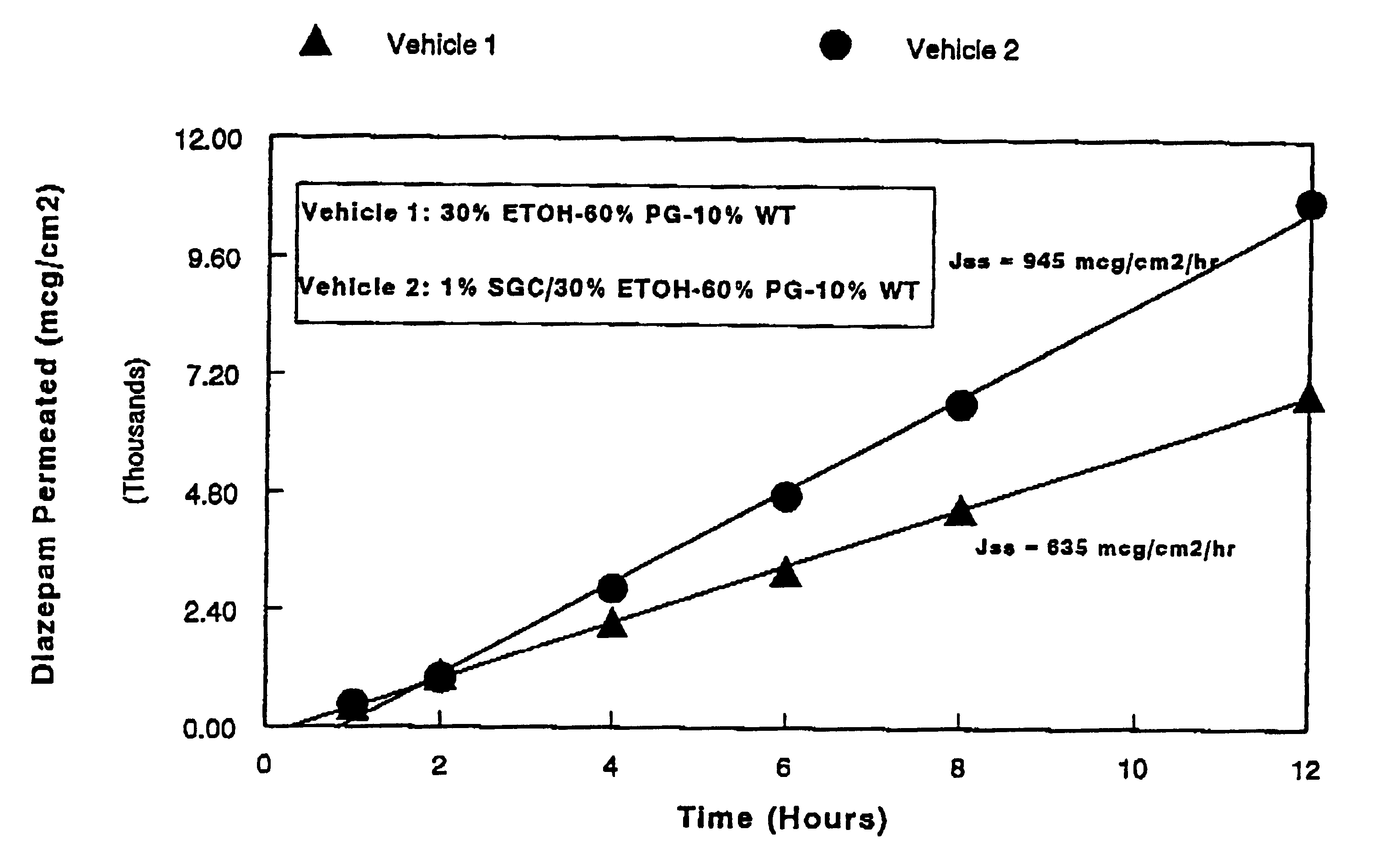
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Melanoma chemoprevention
ActiveUS9393225B2Inhibits melanoma cell growthLowered STAT expressionPharmaceutical delivery mechanismDisease diagnosisEnteral administrationMedicine
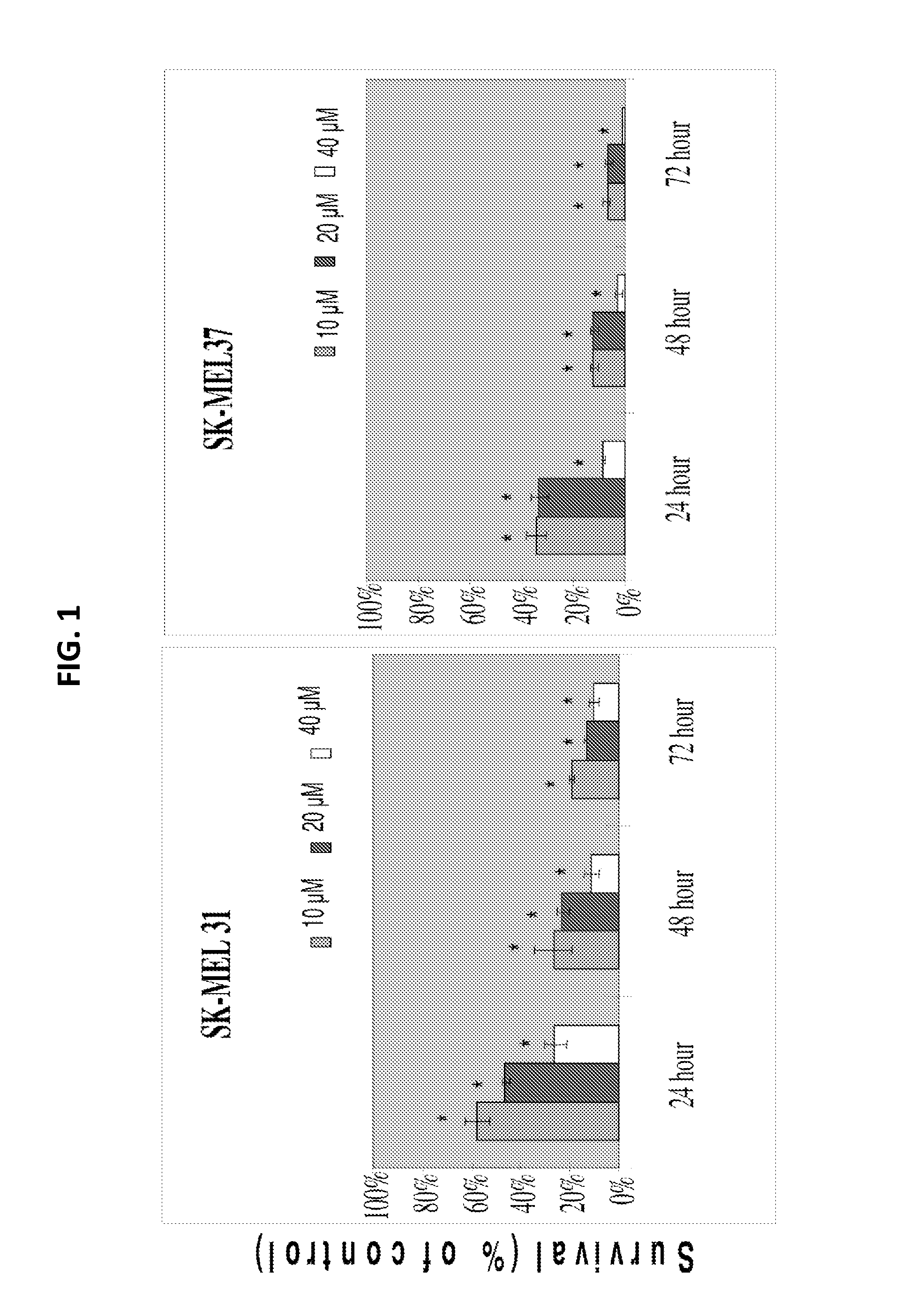
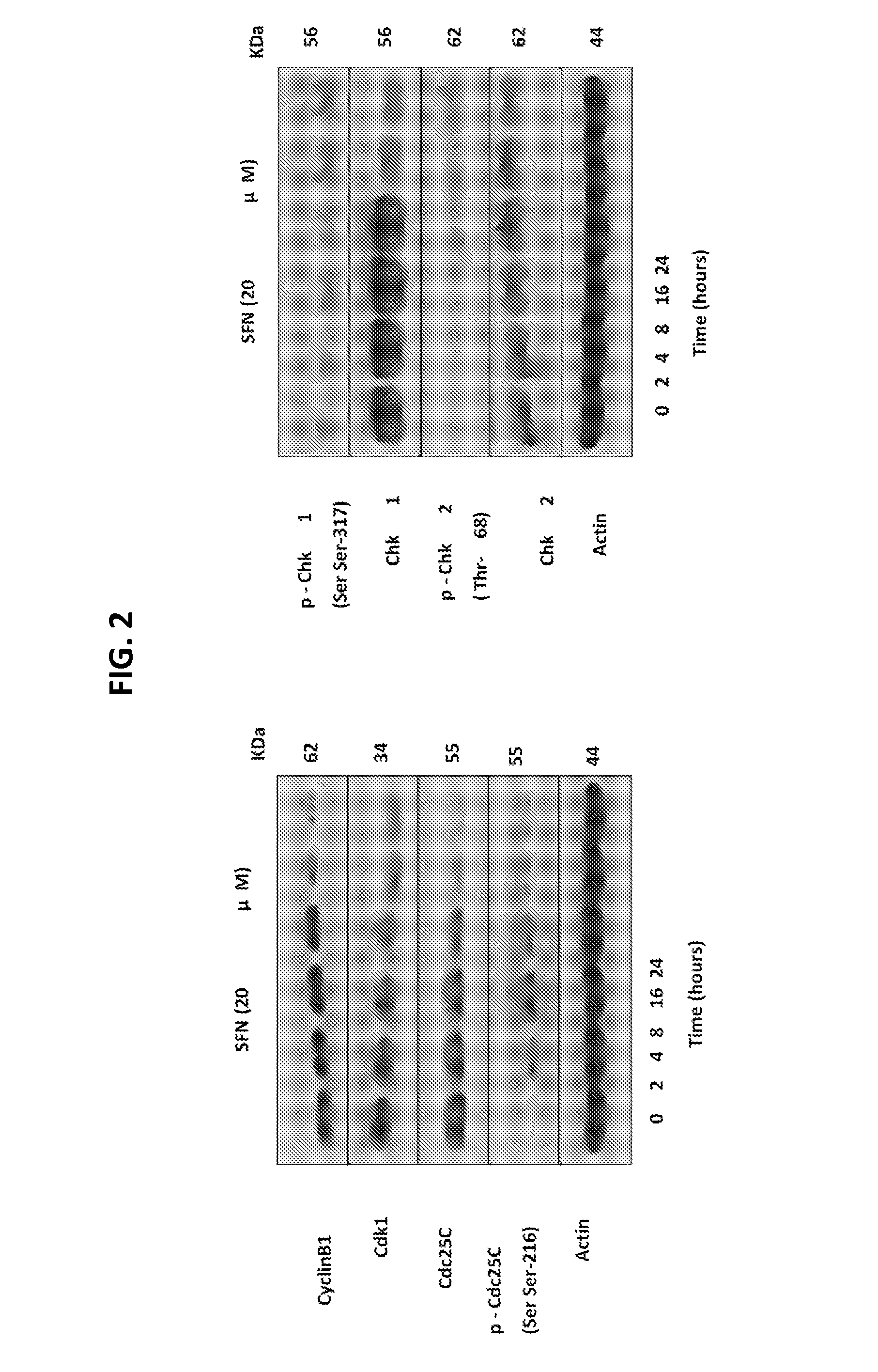
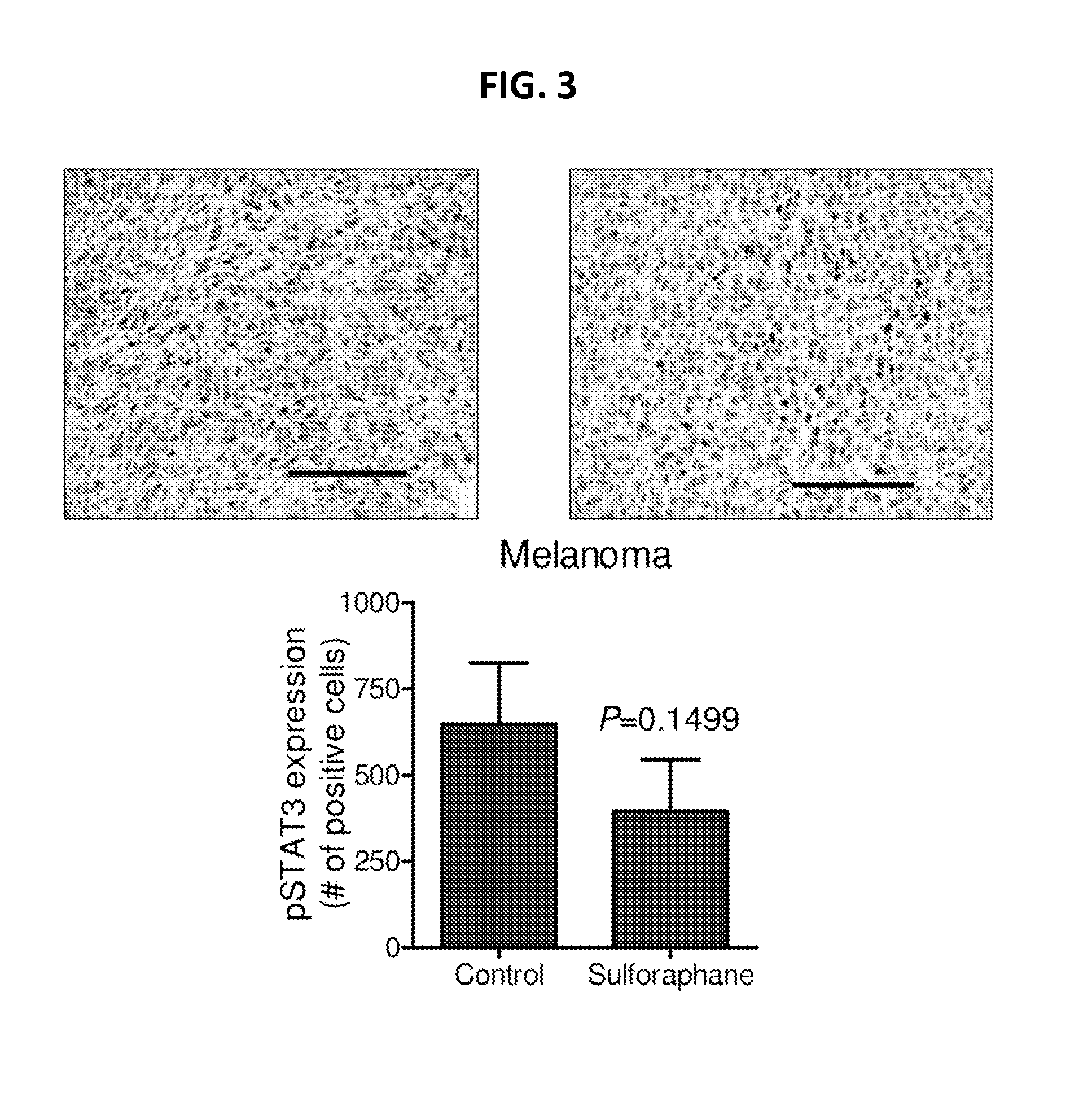
Disclosed herein are methods and uses for preventing melanoma, reducing progression of atypical nevi, and inducing cell cycle arrest and / or apoptosis in a melanoma cell through oral and enteral administration of sulforaphane to subjects indicated to be at risk due to factor(s) such as medical history of atypical nevi, melanoma, or UV exposure. Sulforaphane can be administered orally as a safe and well-tolerated natural agent as a chemopreventive strategy in individuals with atypical melanocytic nevi.
Owner:UNIVERSITY OF PITTSBURGH
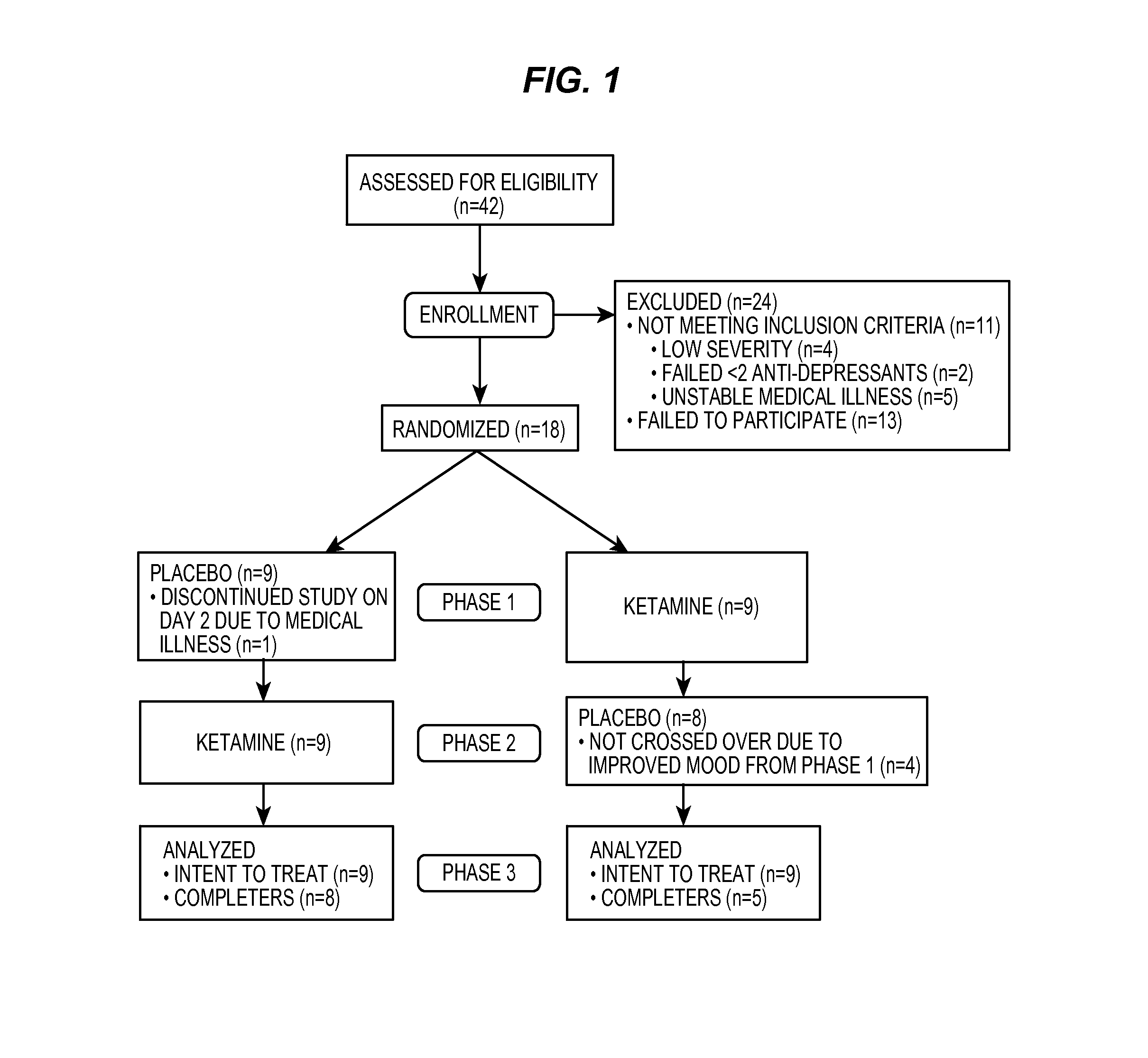
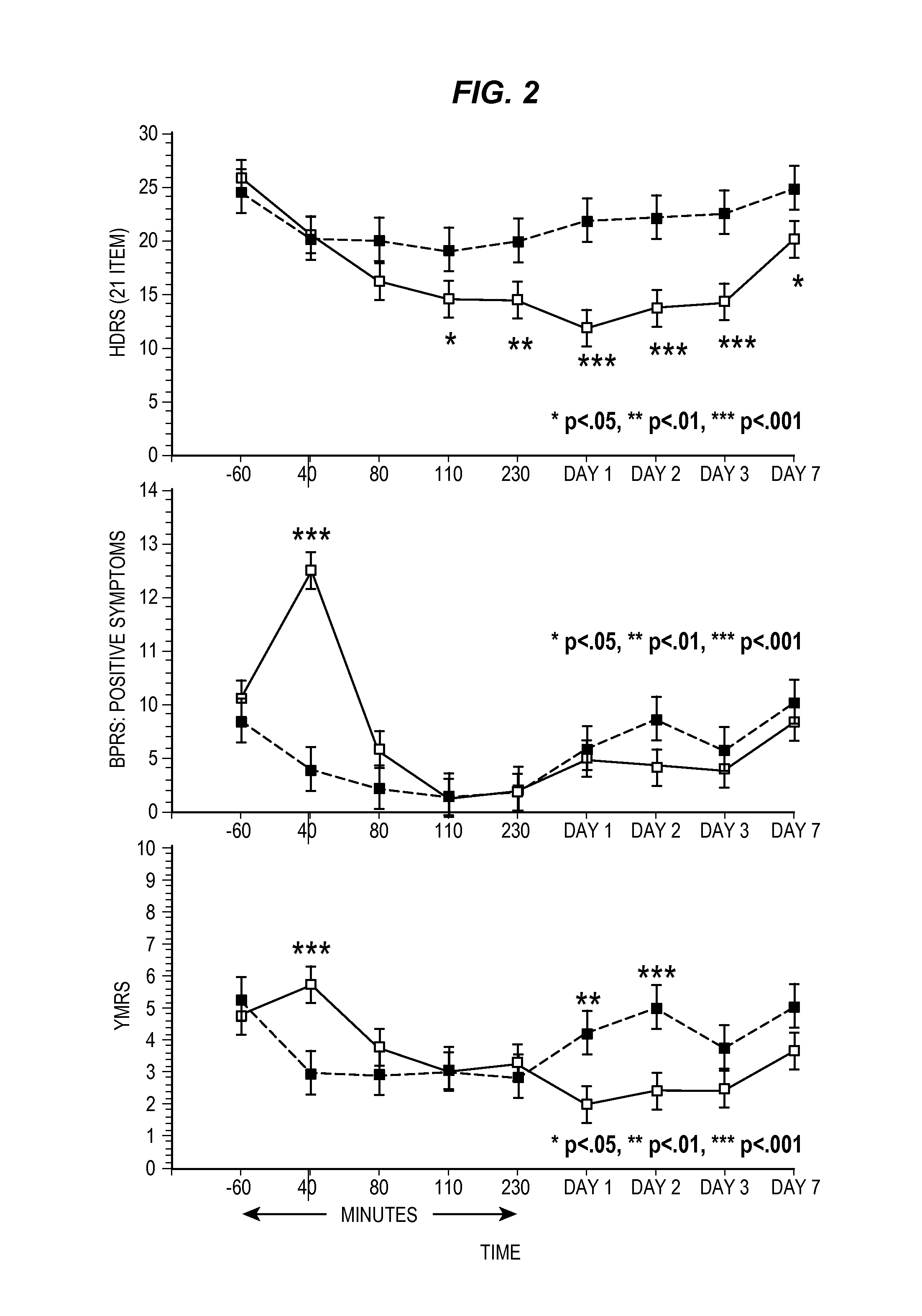
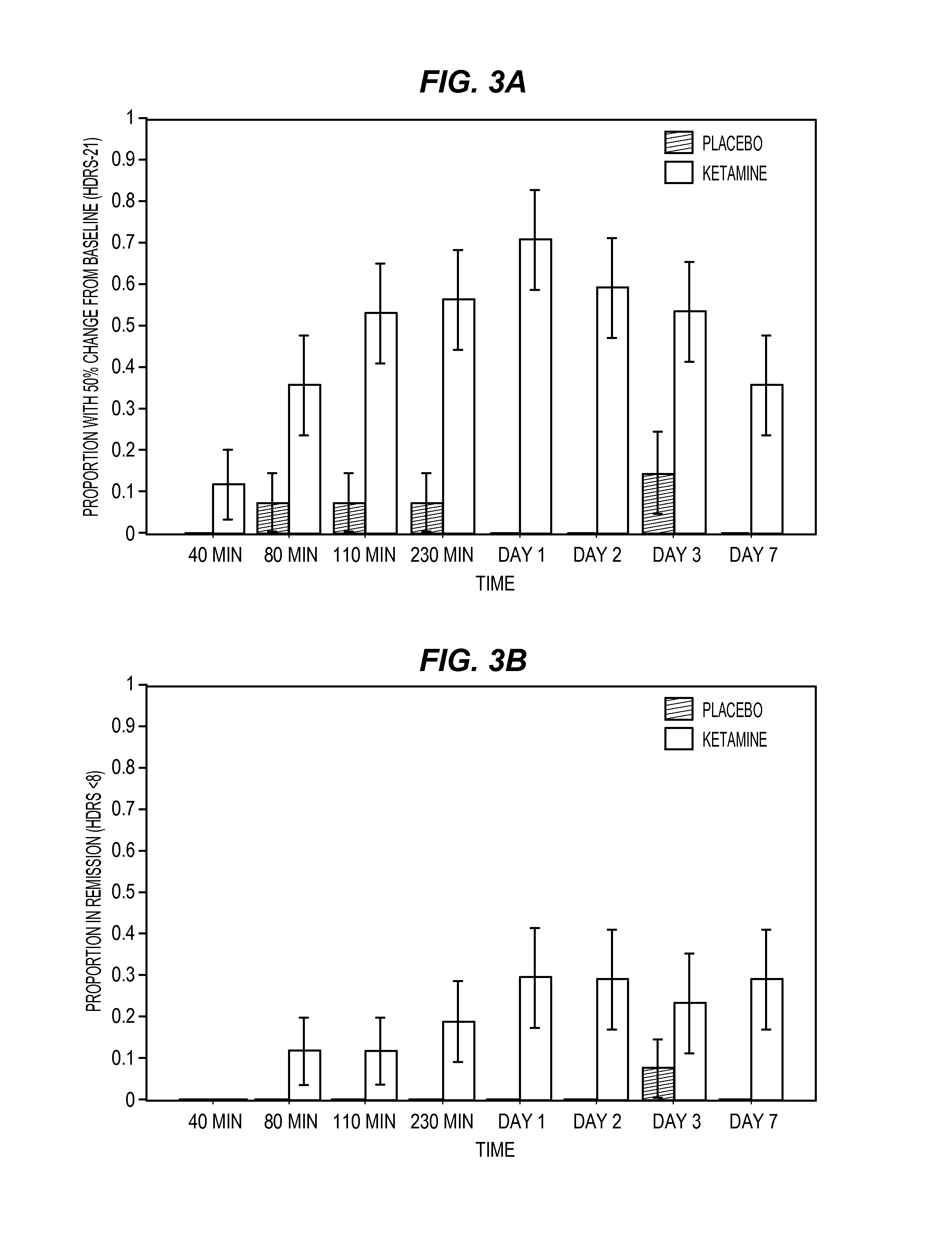
Intranasal administration of ketamine to treat depression
ActiveUS8785500B2Minor adverse side effectAct quicklyBiocideOrganic active ingredientsEnteral administrationKetamine
Methods and compositions for the treatment of treatment-resistant depression are described. More specifically, the invention demonstrates that intranasal administration of ketamine is effective to ameliorate the symptoms of depression in a patient who has not responded to an adequate trial of one antidepressant in the current episode and has recurrent or chronic depressive symptoms (>2 years).
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +3
Method for the treatment of obesity, overweight and fluctuations in blood insuline and/or glucose levels
InactiveUS20030113310A1Low calorific valueLowering indexPeptide/protein ingredientsMetabolism disorderIntestinal structureBlood insulin
The present invention relates to a method of treating or preventing obesity, overweight, fluctuations in blood insulin levels and / or fluctuations in blood glucose levels in mammals. The method acording to the invention comprises the enteral administration to a mammal of an effective amount of a preparation containing an enzyme capable of converting an ingested carbohydrate or digestion product thereof into one or more absorbable components, wherein the total metabolic caloric value of the absorbable component(s) is less than the metabolic caloric value of the ingested carbohydrate or digestion product thereof. Thus the present invention effectively provides a method that allows complete digestion of ingested digestible carbohydrates whilst at the same time reducing the actual metabolic caloric value of said ingested carbohydrates. Another aspect of the invention relates to a pill for oral administration provided with an enteric coating and containing 25 to 10.000 IU glucose isomerase per gram.
Owner:NV NUTRICIA
Matrix-forming composition containing pectin
InactiveUS6884445B2Viscosity is not sufficientGood effectOrganic active ingredientsSugar food ingredientsEnteral administrationShear rate
One aspect of the present invention relates to a liquid edible composition with a pH of more than 6, a viscosity below 600 mPas at a shear rate of 100s−1 and 20° C., and a viscosity of at least 125% of the aforementioned viscosity at a pH below 5 and a temperature of 37° C., the composition comprising at least 0.05 wt. % of pectin having a degree of methoxylation between 2 and 50 and / or of alginate; at least 5 mg calcium per 100 ml; and at least 0.1 wt. % indigestible oligosaccharide having a degree of polymerisation between 2 and 60.Another aspect of the invention relates to a method for the treatment or prevention of overweight or obesity in mammals, said method comprising the enteral administration to a mammal of an effective amount of the aforementioned composition.
Owner:NUTRICIA
Medical operation suture thread
InactiveCN101380484AReduce dosageAchieve long-lasting pain reliefSuture equipmentsAnalgesics drugsEnteral administration
The invention discloses a medical suture that contains an analgesic drug which can slowly release in a body. The suture is combined with the analgesic drug. The medicine administration is simultaneously accomplished during suture, and the medicine is slowly released from the suture, thereby achieving the long term acesodyne effect on the sutured part. Due to the partial medicine administration, the medicine dosage is greatly reduced compared with the whole body medicine administration, and the side effect on the whole body is remarkably reduced while the partial effective pain stop is achieved.
Owner:李捷
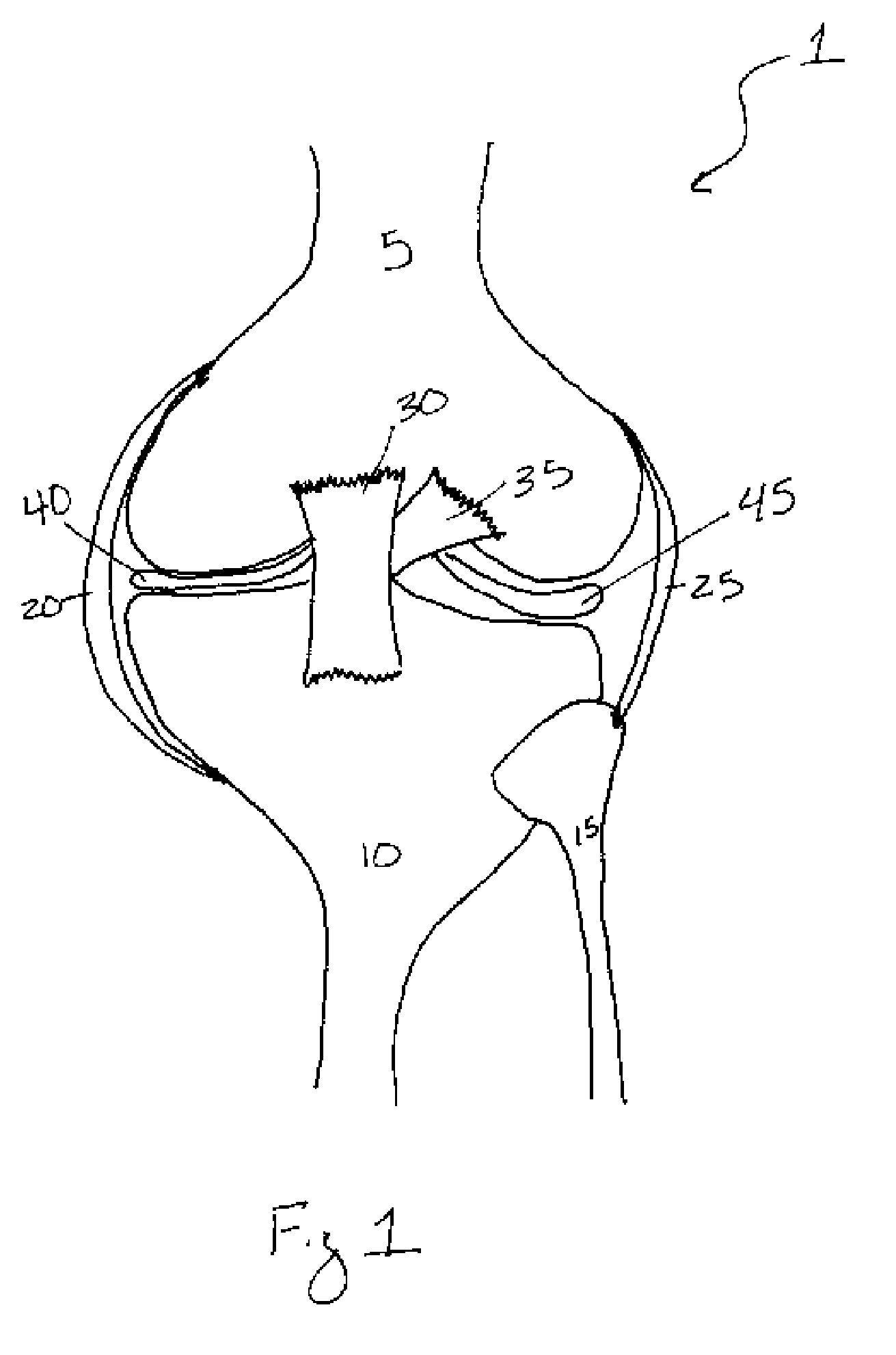
Enteral formulation
Owner:ERNEST STEPHEN P
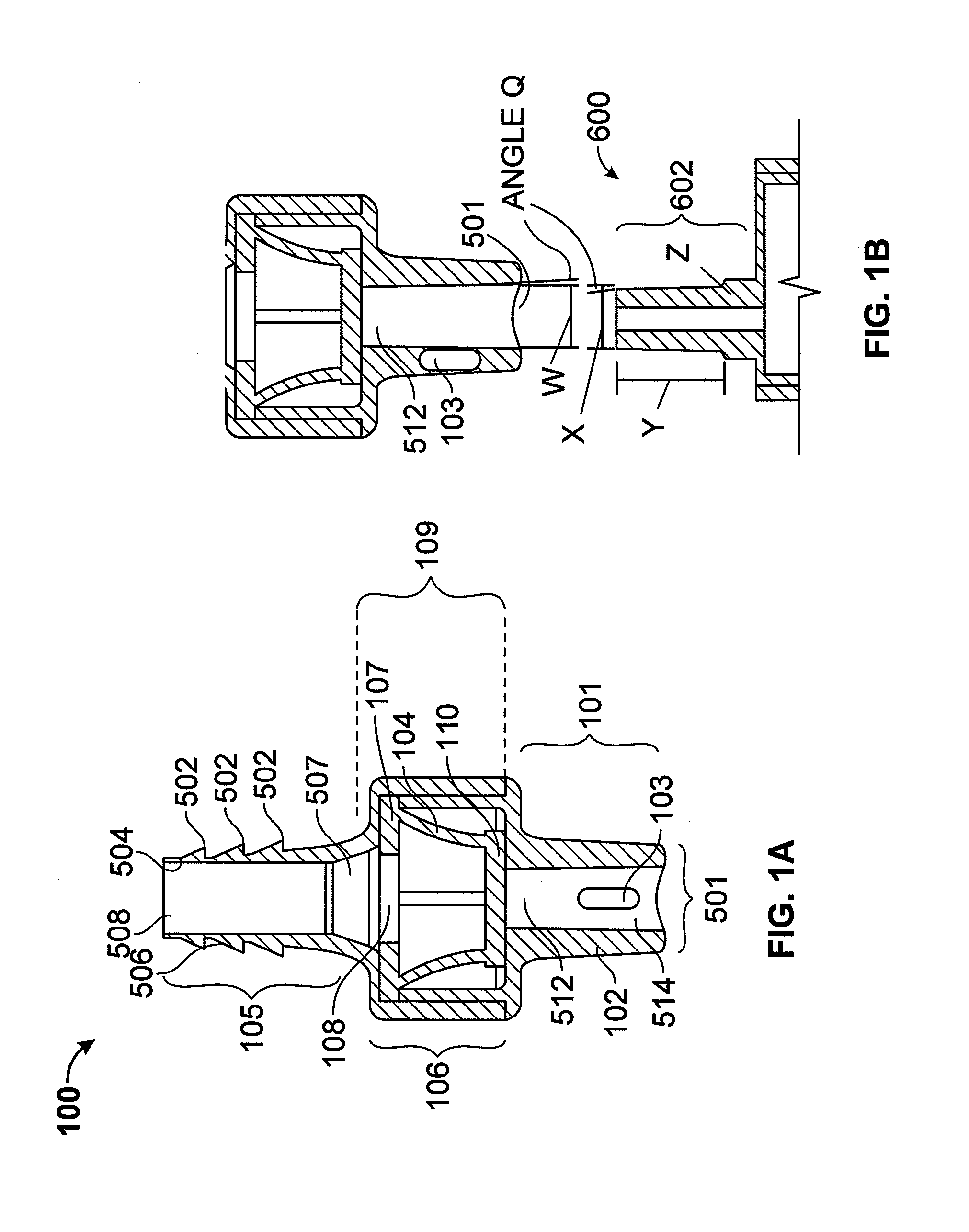
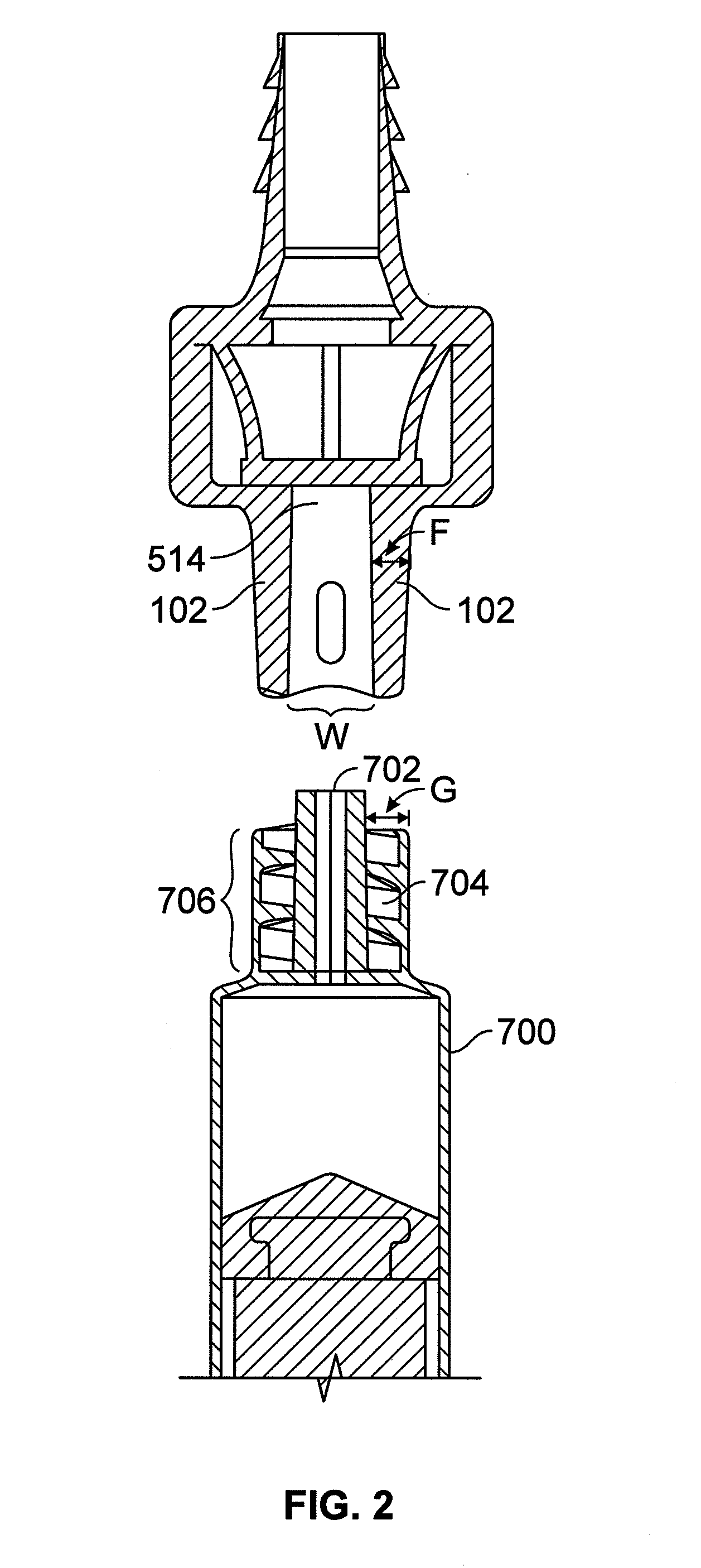
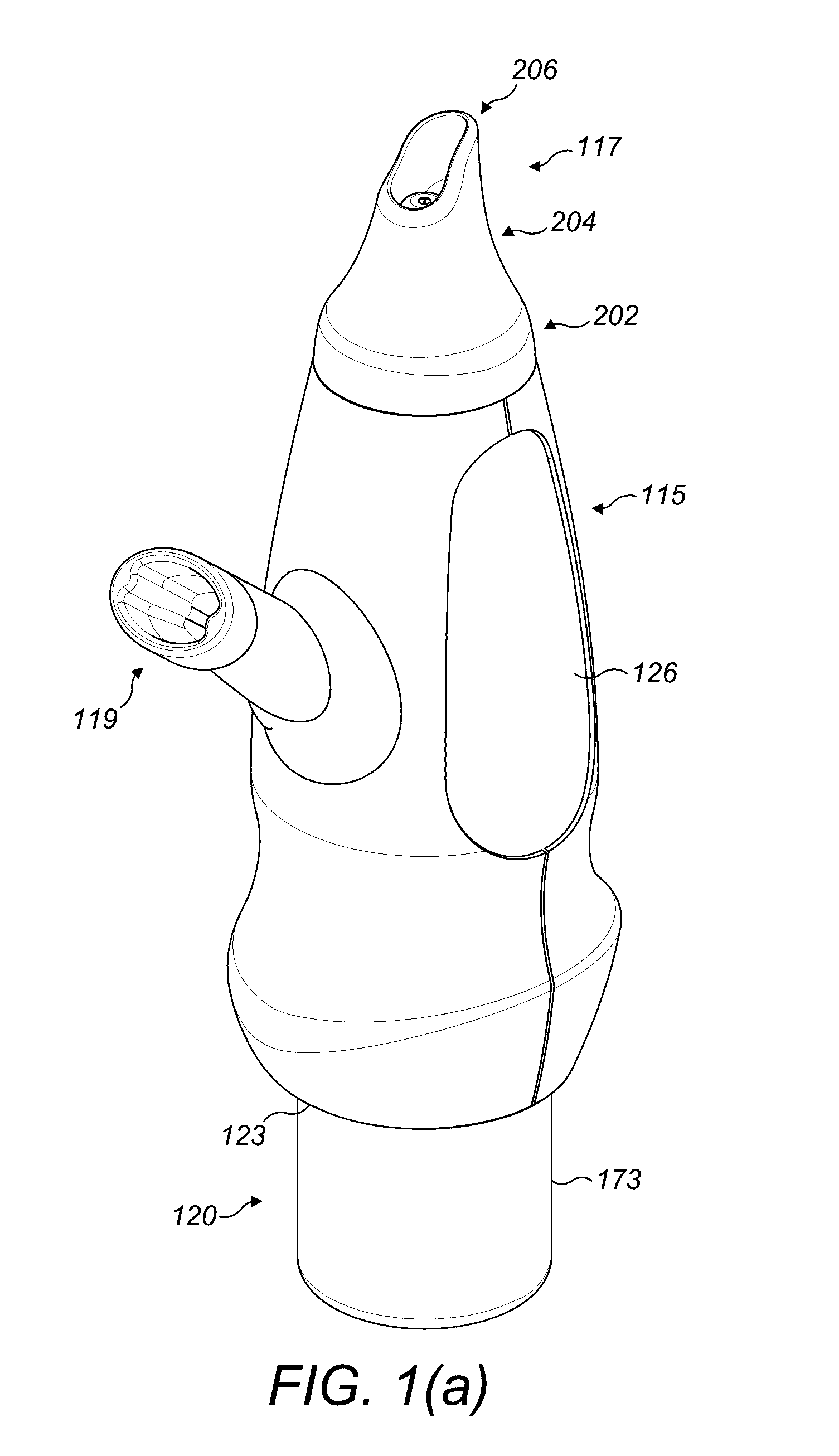
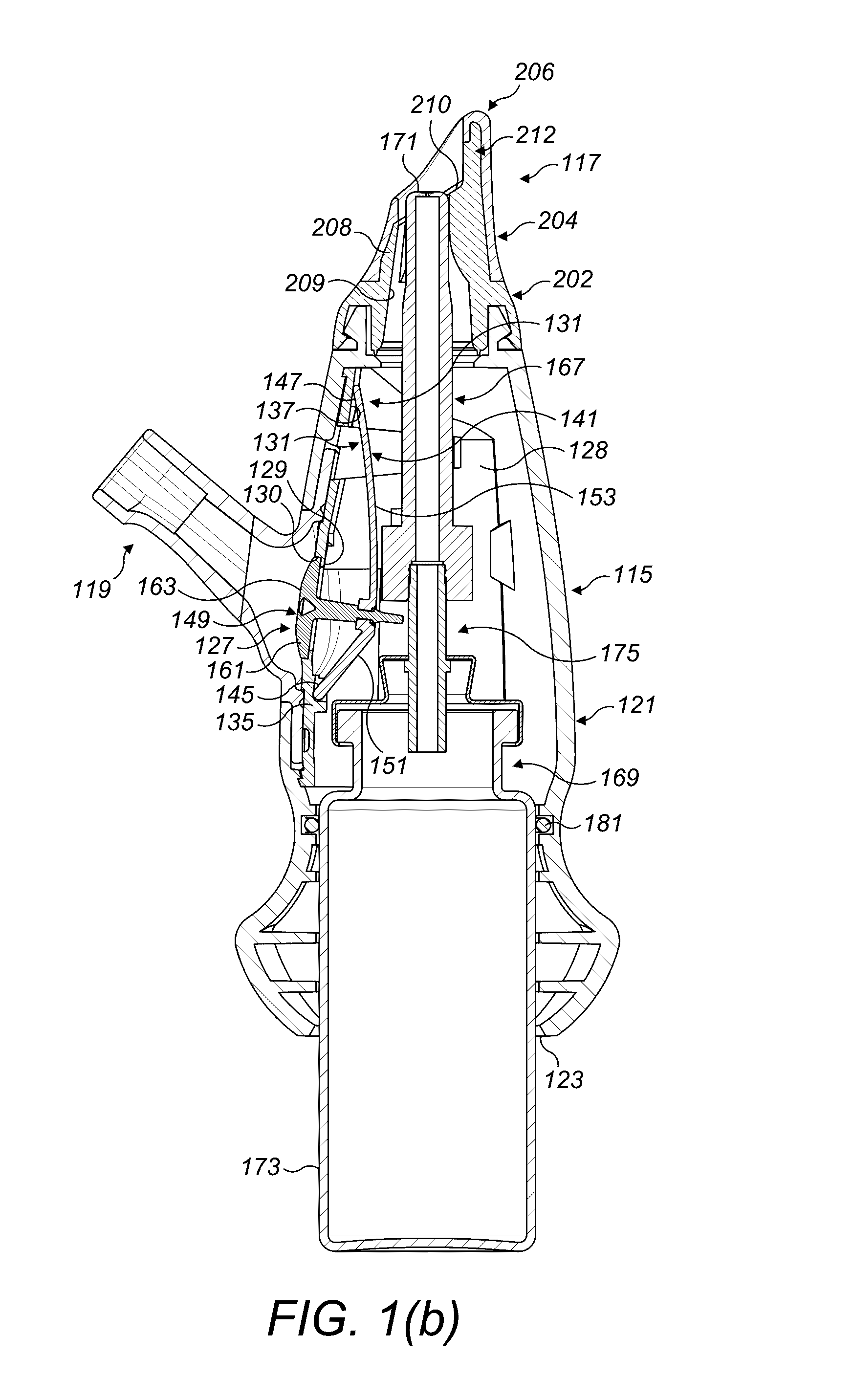
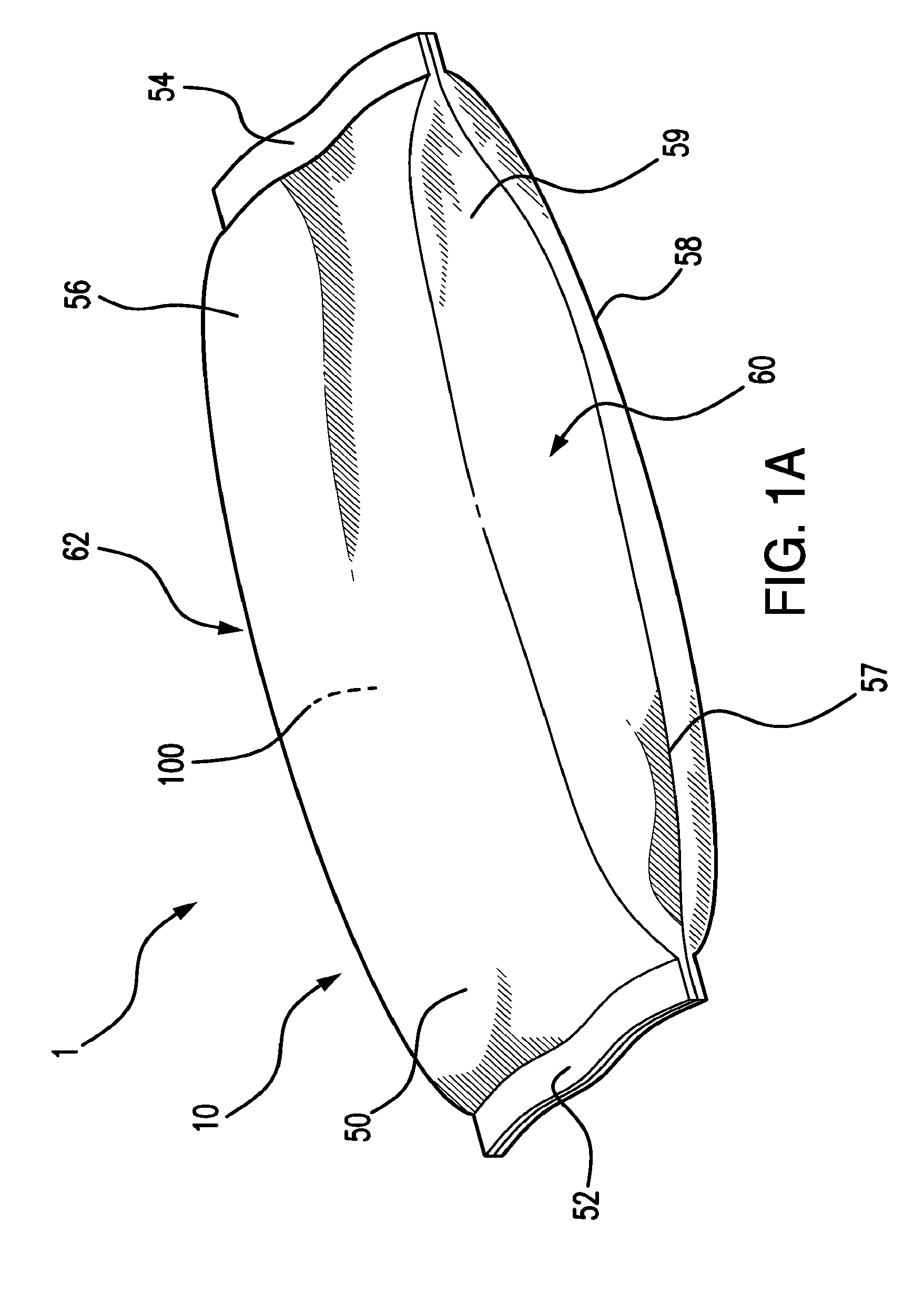
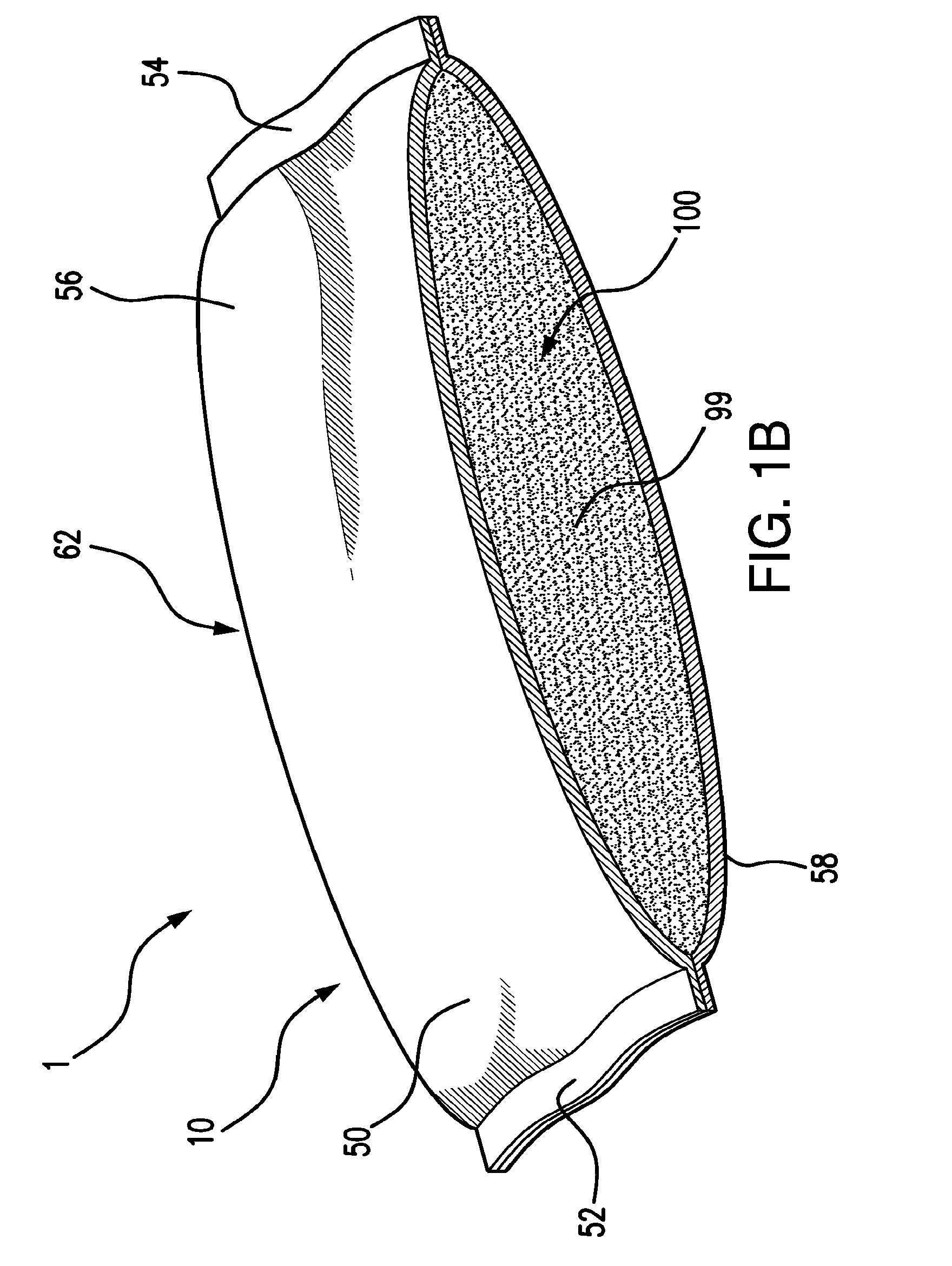
Valved enteral administration assembly
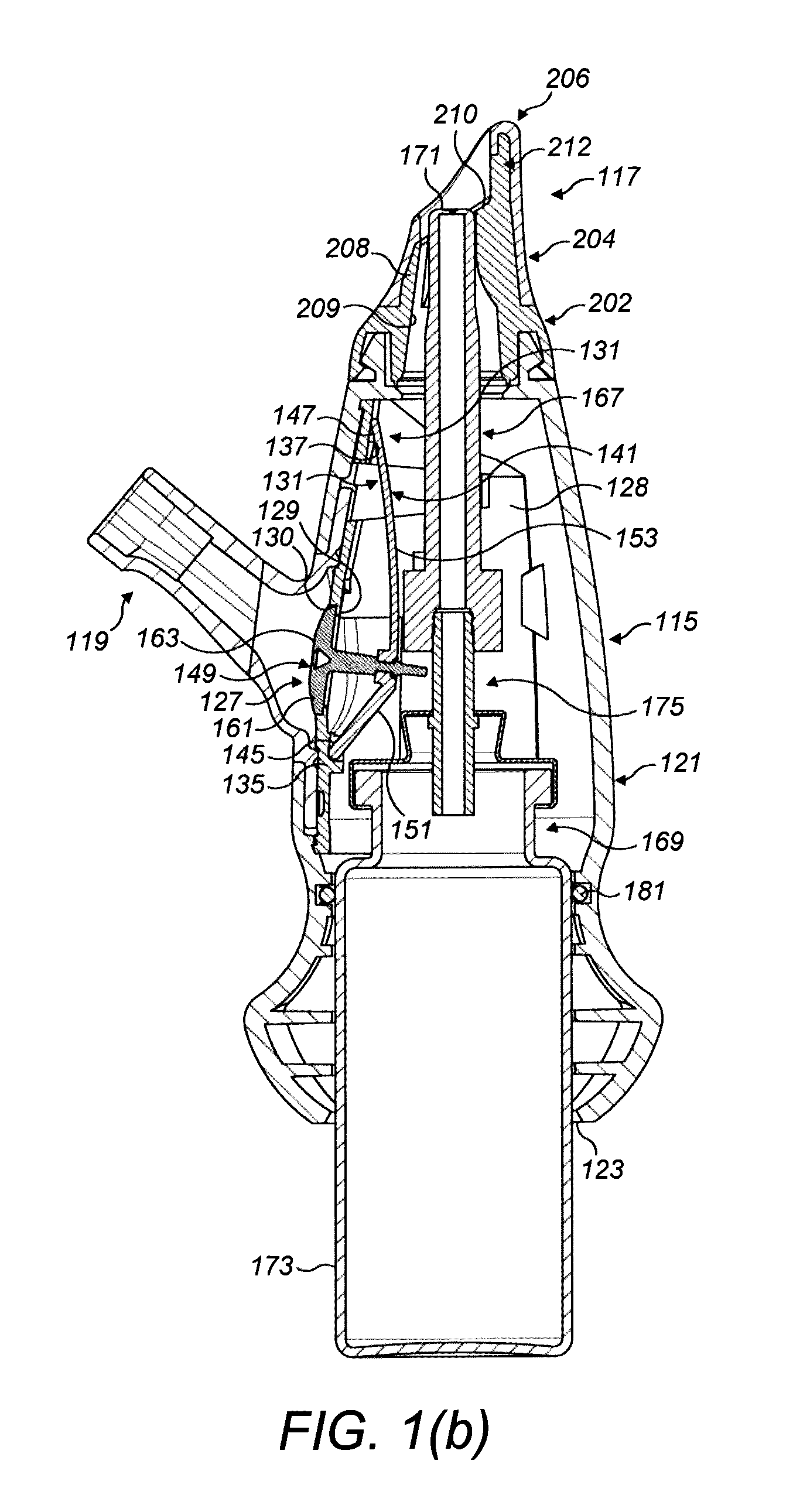
ActiveUS20150045746A1Safety managementReduces biofilm formationMedical devicesOral administration deviceEnteral administrationEngineering
An enteral administration assembly for administering fluids through an enteral device. The administration assembly comprising an inlet, an outlet, and a valve mechanism between the inlet and outlet. The inlet is configured to interlock with only an enteral device to form a sealed connection allowing the passage of fluid between the enteral device and the inlet. The assembly may include a valve or turbulating mechanism comprising a valve and at least one turbulator adapted to change the direction of fluid flowing through the assembly to prevent clogging and flocculation of administered substances.
Owner:HOSPI CORP
Intranasal Administration
ActiveUS20160310683A1Easy to insertIncrease the opening areaNervous disorderPeptide/protein ingredientsNasal cavityEnteral administration
A delivery device for and method of modulating a condition relating to sodal cognition and / or behaviour in a human subject using oxytocin, non-peptide agonists thereof and / or antagonists thereof, comprising: providing a nosepiece to a first nasal cavity of the subject; and providing a supply unit for administering less than 24 IU of oxytocin, non-peptide agonists thereof and / or antagonists thereof through the nosepiece to an upper region posterior of the nasal valve which is innervated by the trigeminal nerve.
Owner:OPTINOSE AS
Dosage forms for oral administration and methods of treatment using the same
InactiveUS20130243871A1Increase intakeEffective and prolonged treatmentOrganic active ingredientsPowder deliveryEnteral administrationOral medication
Owner:NEOS THERAPEUTICS LP
Androgen composition for treating an opthalmic condition
ActiveUS20120190661A1Suitable for topical administrationSenses disorderAntipyreticDiseaseEnteral administration
The disclosure provides compositions for treating an ocular condition. The composition comprises a physiologically effective amount of an androgen, wherein the composition is suitable for topical administration to an eye. The disclosure further provides methods for treating an ocular condition with the disclosed compositions.
Owner:ALLERGAN INC
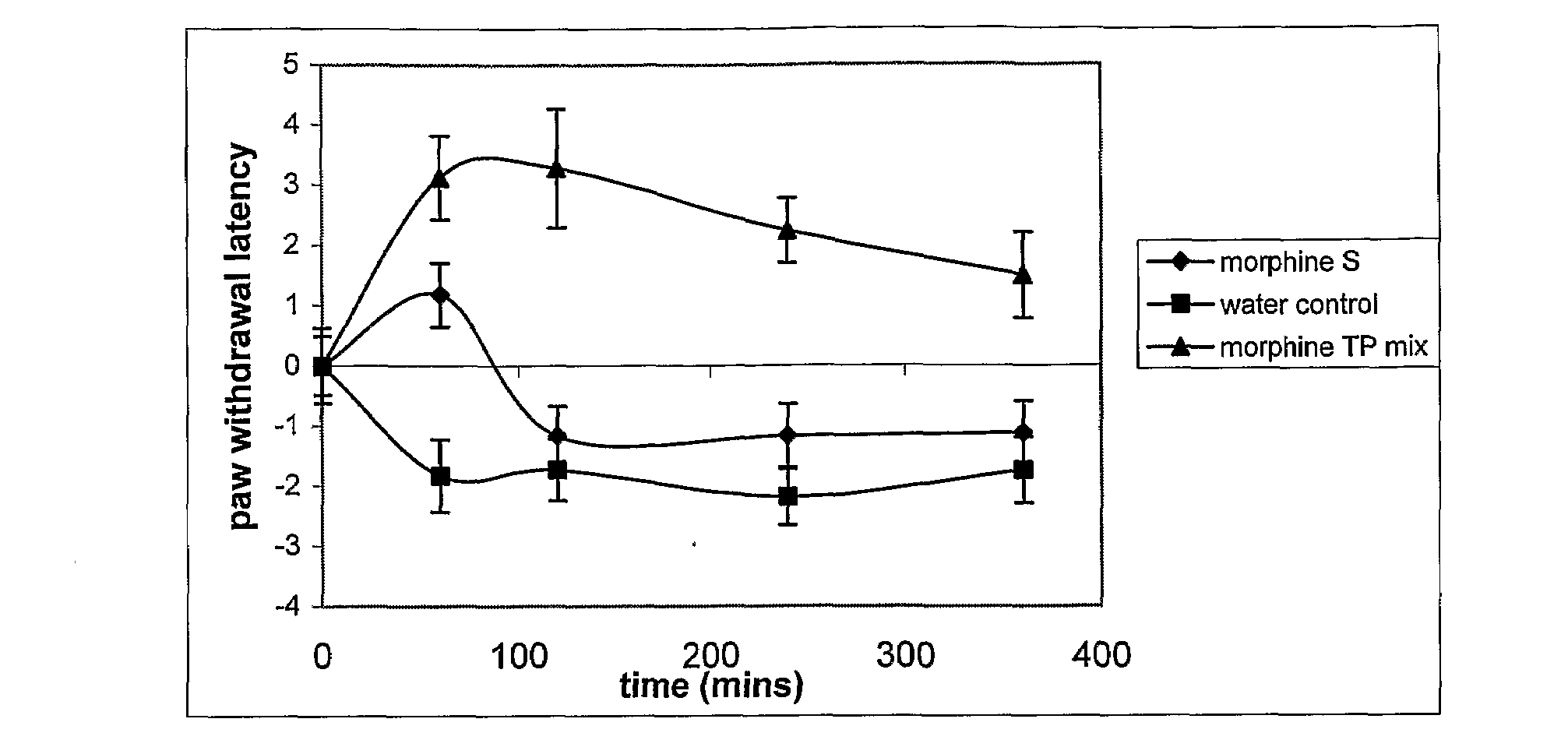
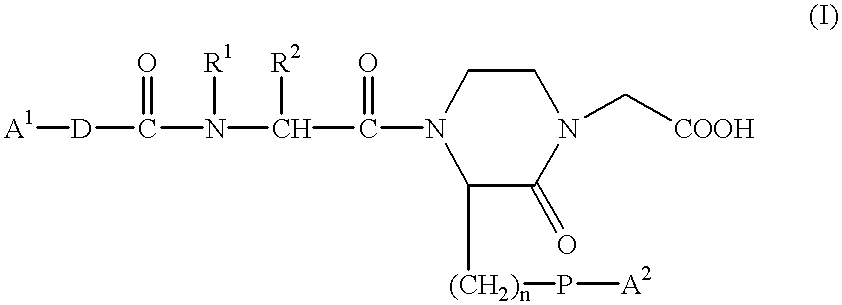
Carrier For Enternal Administration
InactiveUS20090004166A1Good curative effectImproving efficacy and transportBiocideNervous disorderEnteral administrationMedicine
There is provided a carrier for use in enteral administration of biologically active compounds, said carrier comprising an effective amount of one or more phosphate derivatives of one or more electron transfer agents.
Owner:VITAL HEALTH SCIENCES PTY LTD
Intranasal Administration
ActiveUS20160331916A1Easy to insertIncrease the opening areaNervous disorderPeptide/protein ingredientsNasal cavityEnteral administration
A nosepiece for delivering substance to a nasal cavity of a subject, the nosepiece comprising a body part which comprises a base portion which defines a flow passage therethrough, and a projection at a distal end of the base portion which at least in part provides a tip of the nosepiece and confers a rigidity in the sagittal direction, which enables the tip to open fleshy tissue at an upper region of the nasal valve and thereby expand an open area of the nasal valve, and a flexibility in a lateral direction, orthogonal to the sagittal plane, which facilitates insertion of the tip into the nasal valve.
Owner:OPTINOSE INC
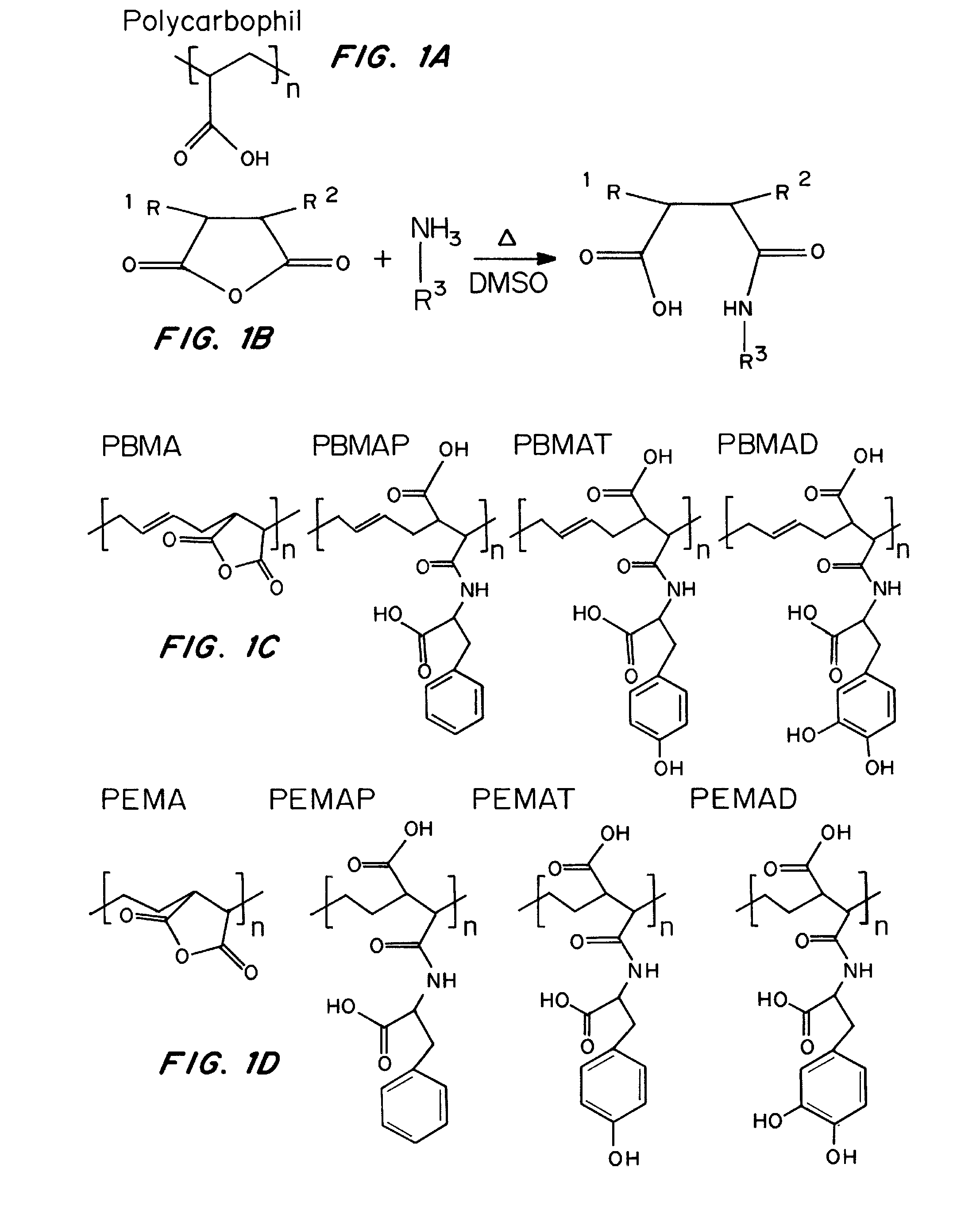
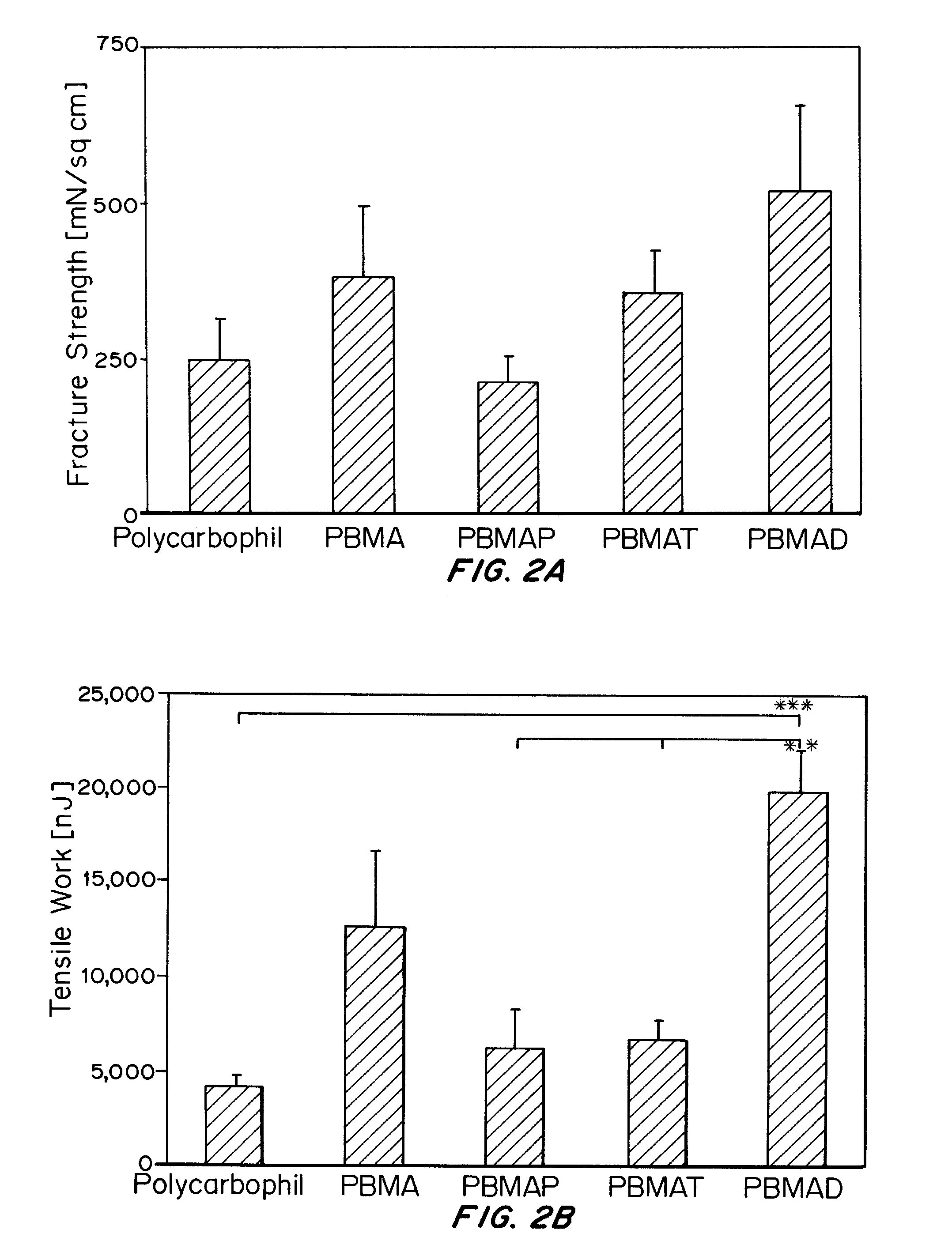
Nanoparticle compositions and methods for improved oral delivery of active agents
ActiveUS20120009267A1Minimize burst effectPromote absorptionOrganic active ingredientsBiocideEnteral administrationOral medication
Nanoparticles, compositions, and methods for the improved uptake of active agents are disclosed herein. The compositions contain a monodisperse population of nanoparticles, preferably including an active agent, where the nanoparticles are formed from a polymeric material possessing specified bioadhesion characteristics. Following enteral administration, preferably oral administration, the nanoparticles exhibit total intestinal uptakes of greater than 20%, preferably greater than 45%, more preferably greater than 65%. When compared to uptake of the same composition in the absence of the bioadhesive polymeric material, the nanoparticles have significantly increased uptake with intestinal uptake of the increased by more than 100%, preferably even greater than 500%. Further disclosed herein is a method of producing multi-walled nanoparticles, as well as methods of using thereof. Multi-walled particles prepared using the method disclosed herein are useful for controlling the release of active agents.
Owner:BROWN UNIVERSITY
Pharmaceutical compositions of acetylsalicylic acid and omega-3 oils
InactiveUS20030199481A1Safe and reliable administrationDevelopment actionBiocideSalicyclic acid active ingredientsEnteral administrationOral medication
The present invention provides pharmaceutical compositions of acetylsalicylic acid and omega-3 oils which enable safe and stable oral administration within the range of the narrow therapeutic index prescribed for the prevention of alterations of the cardiovascular system.
Owner:ALTERGON
Solid composition for the oral administration of dyes and diagnostic use thereof
ActiveUS8545811B2Powder deliveryLuminescence/biological staining preparationEnteral administrationOral medication
The present application discloses solid compositions for the oral administration of dyes, and diagnostic use thereof. Preferably, such diagnostic use is aimed at the diagnostic evaluation of the gastrointestinal tract.
Owner:COSMO TECH LTD
Treatment of obesity using non-daily administration of 6-O-(4-dimethylaminoethoxy) cinnamoyl fumagillol
The invention generally relates to methods of treating an overweight or obese subject, and treating overweight- or obesity-related conditions using non-daily administration of e.g., a MetAP-2 inhibitor.
Owner:ZAFGEN
Methods of treating a trauma or disorder of the knee joint by local administration and sustained-delivery of a biological agent
InactiveUS7910123B2Good treatment effectEliminate needPeptide/protein ingredientsDiagnosticsKnee JointLigament structure
Methods and apparatus of providing a subject with post-operative, sustained-release of a biological agent within a synovial joint is disclosed. These methods involve securing a depot containing the biological agent to a ligament, tendon, muscle within the joint to provide sustained-release of the agent while allowing for normal joint articulation. This methodology may be utilized to provide for sustained-release of a biological agent useful in treating various traumas and disorders of the joint. Such biological agents include antagonists of inflammation-related proteins, such as TNF-α, IL-1β, IL-6, IL-8, NF-κB, High Mobility Group Box 1 (HMG-B1), IL-2, IL-15 and steroidal and non-steroidal anti-inflammatories. Other biological agents include anti-inflammatory cytokines such as IL-10, IL-4, IL-13, and TGF-β. The biological agents also include osteogenic and cartilage producing growth factors such as, but not limited to, BMP-2, BMP-4, BMP-6, BMP-7, BMP-8, and MIA CD-RAP. Finally, the biological agents include siRNA and / or therapeutic antibodies.
Owner:WARSAW ORTHOPEDIC INC
Tea Composition For Oral Administration
InactiveUS20160324777A1To promote metabolismPrevent arthritisDispersion deliveryPre-extraction tea treatmentEnteral administrationOral medication
This invention concerns an enhanced green tea based product for oral use whereby the nutritional and health benefits of green tea, and additives which shall include some combination of flavoring, preservatives, caffeine, humectants, and water, can be ingested and absorbed by the user.
Owner:POCKET TEA
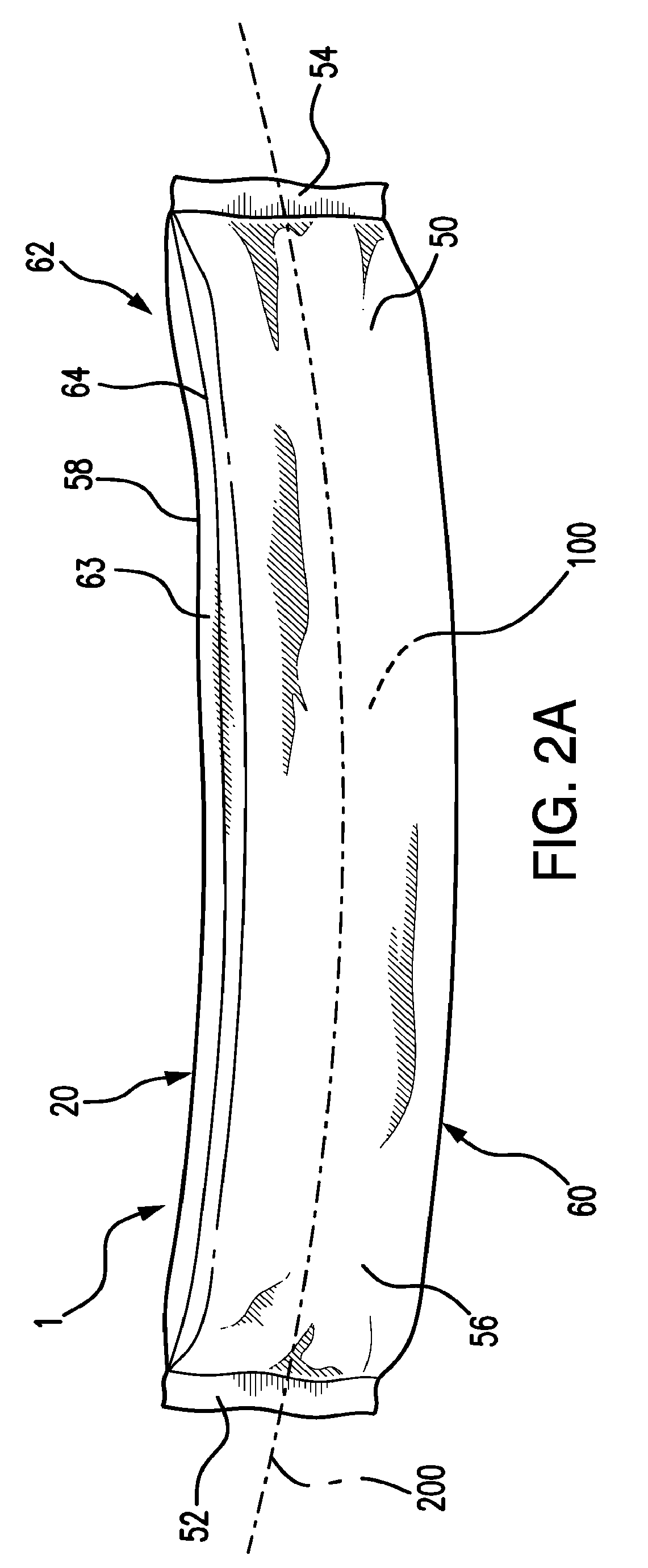
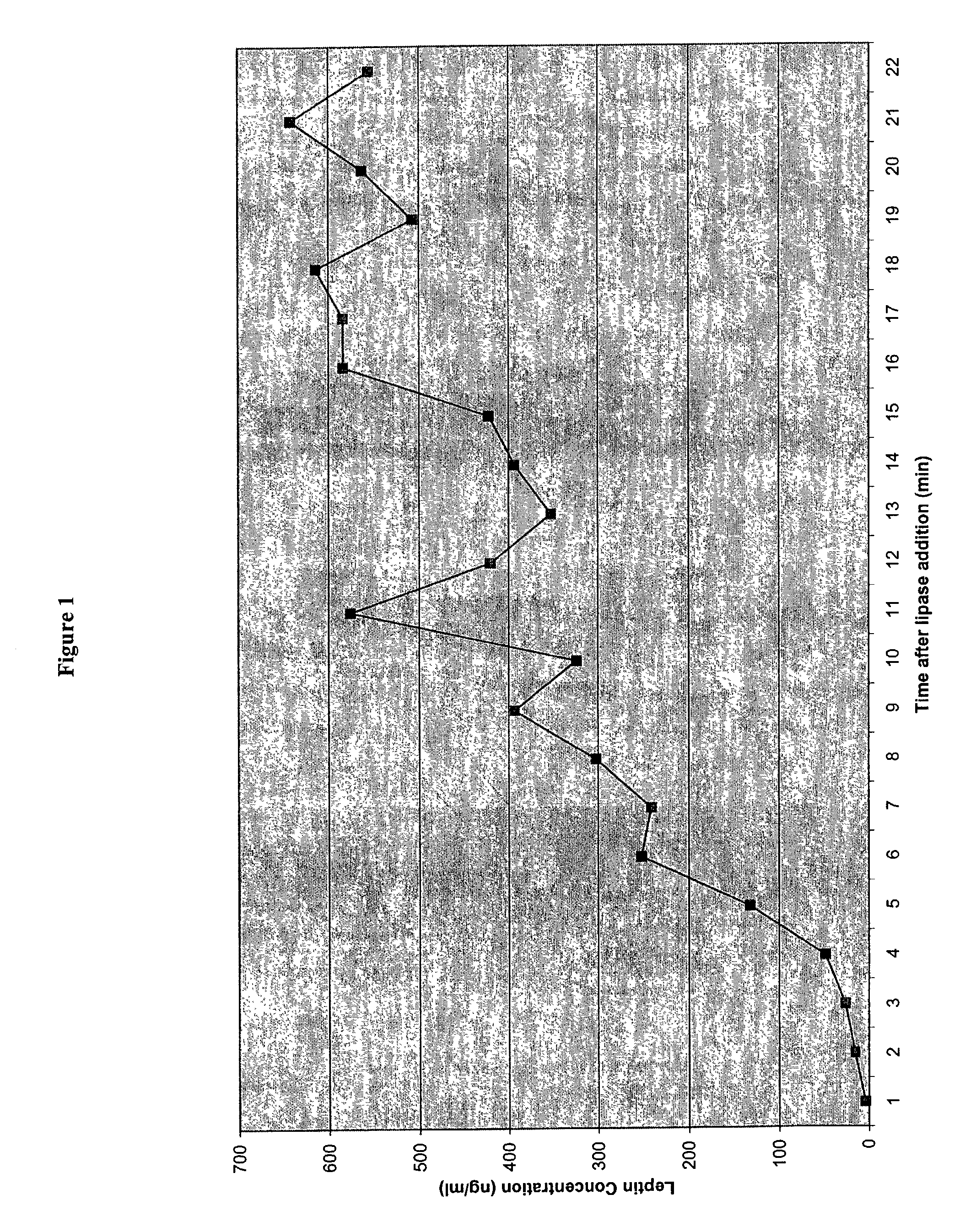
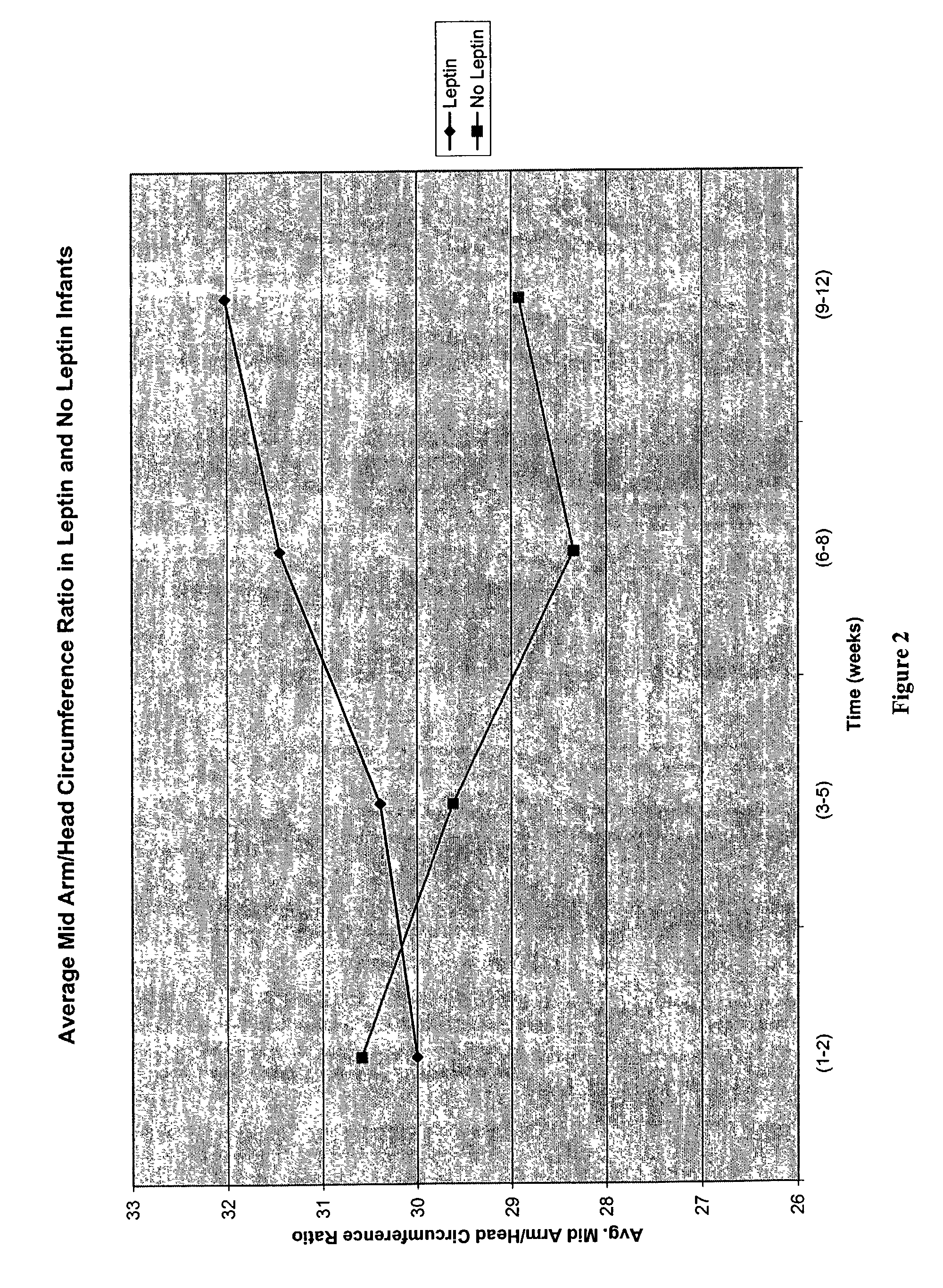
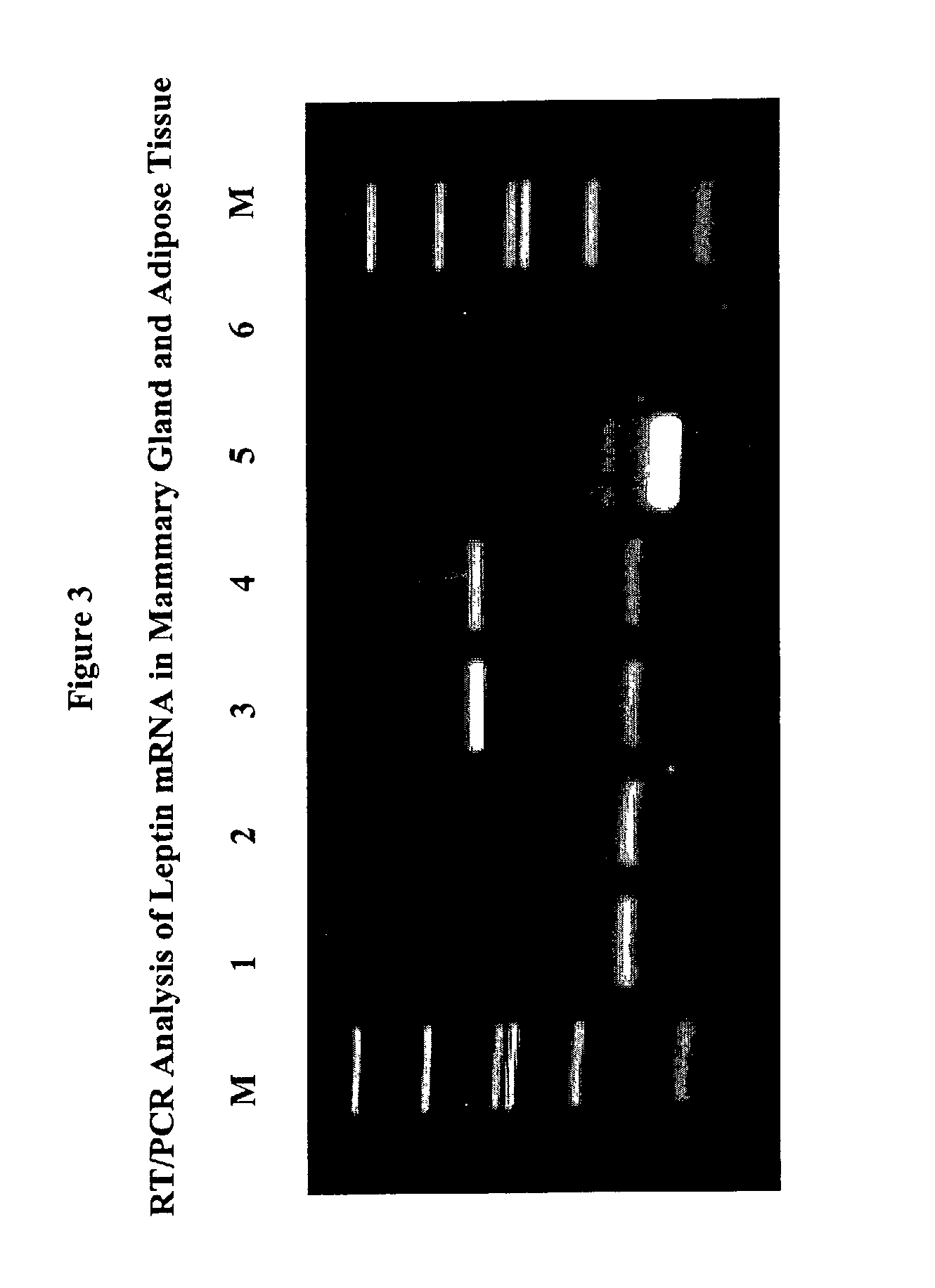
Administration of leptin
A method and composition for administering leptin to a subject. The invention includes suspending isolated native leptin-containing milk fat globules in a suitable medium for administering to a subject. The suspended milk fat globules may be administered orally as well as by intravenous, intramuscular, intraperitoneal, other enteral routes of administration, and other parenteral routes of administration. The invention includes a method for treating growth or maturational-related disorders in newborns as well as subjects having conditions that can be treated by the administration of leptin.
Owner:THE NEMOURS FOUND
Method for preparing plane hollow microneedle for transdermal administration
InactiveCN101905856ATo achieve the purpose of sustained releasePrecise deliveryDecorative surface effectsMicroneedlesEnteral administrationChannel pattern
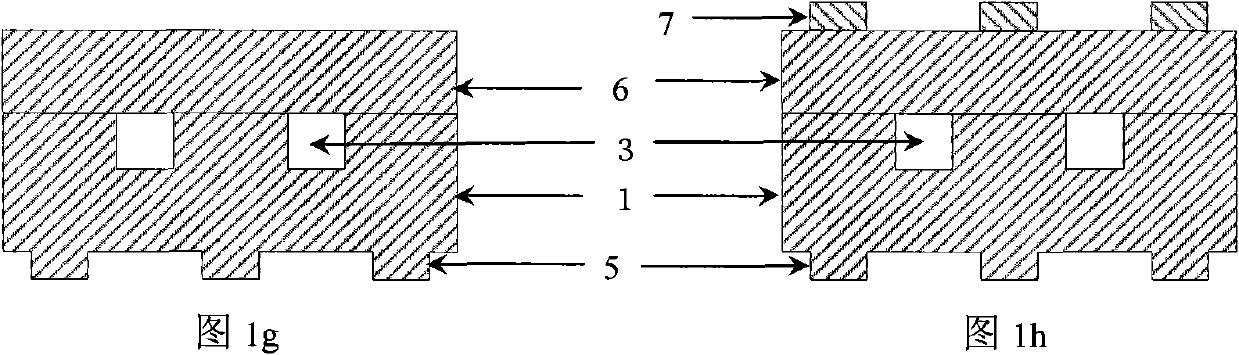
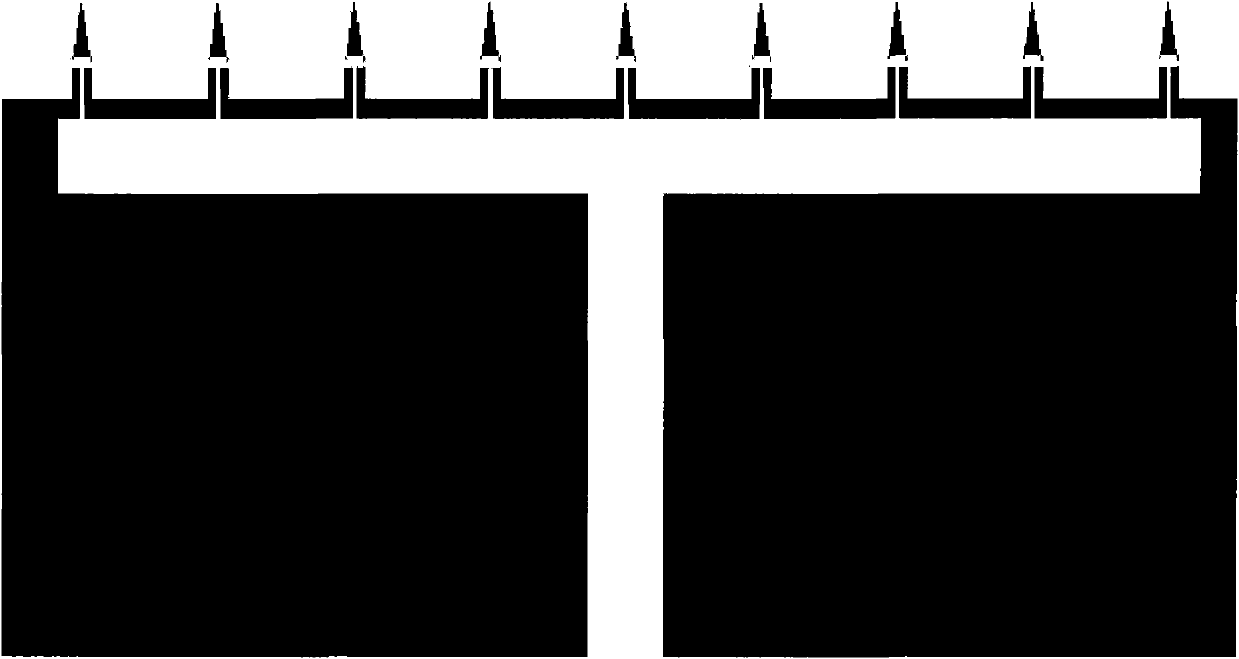
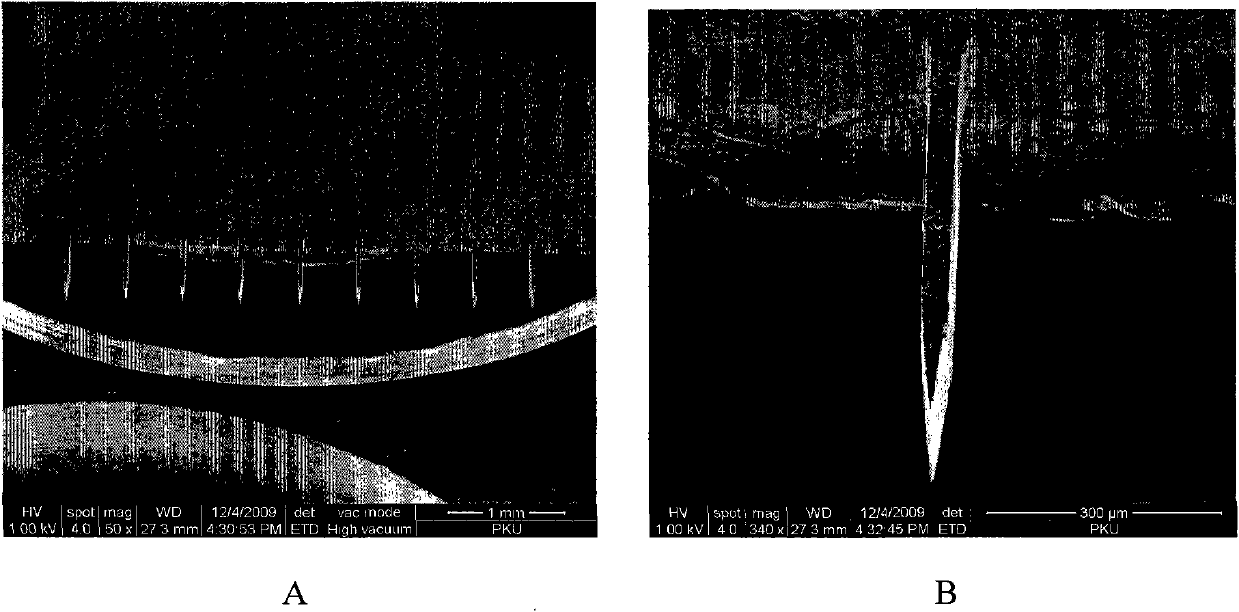
The invention discloses a method for preparing a plane hollow microneedle for transdermal administration, which comprises the following steps of: defining a microneedle flow channel pattern on one surface of a first metal substrate to form a trench, and defining a microneedle pattern on the other surface to form a cutting sign, wherein the central axis of the flow channel pattern is aligned with that of the microneedle pattern; bonding a second metal substrate with a trench surface of the first metal substrate; thinning a non-bonding surface of the second metal substrate, and allowing the cutting sign on the first metal substrate to align with and transfer to the non-bonding surface of the second metal substrate; and thinning a non-bonding surface of the first metal substrate, and cutting by aligning with the cutting sign on the second metal substrate to form the plane hollow microneedle. Based on the micromachining technology and by combining the conventional technology of machining and cutting at the same time, the method reduces the process difficulty, increases the using reliability of the microneedle, and is favorable for quantity production.
Owner:PEKING UNIV
Transnasal anticonvulsive compositions and modulated process
InactiveUS7132112B2Promote absorptionIncrease permeationNervous disorderAerosol deliveryNasal cavityCo administration
A novel method of vehicle modulated administration of an anticonvulsive agent to the mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol (10–80%) or a glycol (10–80%), and their combinations with a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Cyclised alpha-conotoxin peptides
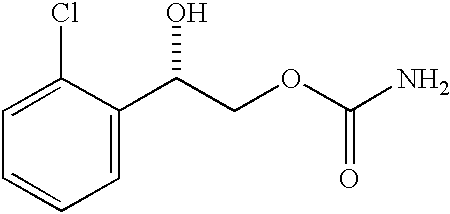
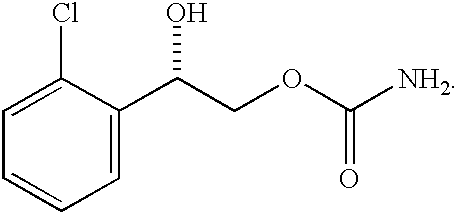
This invention relates to an oral or enteral pharmaceutical preparation comprising at least one synthetically cyclised alpha-conotoxin peptide having an amide cyclised backbone such that the peptide has no free N- or C- terminus, said peptide having the ability to inhibit a nicotinic acetylcholine receptor and comprising four cysteine residues bonded in pairs to form two disulfide bonds, wherein the N-terminus of the corresponding linear / non-cyclised conotoxin peptide is linked to the C-terminus by a peptide linker, in a vehicle which is pharmaceutically suitable for oral or enteral administration.
Owner:THE UNIV OF QUEENSLAND
Method for transdermal administration of GP IIb/IIIa antagonist
InactiveUS6322550B2Maintain stabilityImprove efficacyOrganic active ingredientsElectrotherapySide effectLower risk
A method for transdermal administration of a GP IIb / IIIa antagonist by iontophoresis, comprising plural electric current application steps, progressively reduced in current density. The method insures excellent pharmacologic efficacy with a low risk for side effects in the prevention and therapy of (1) angina pectoris, (2) unstable angina and (3) ischemic complications and coronary arterial reocclusion or restenosis associated with PTCA or coronary thrombolysis.
Owner:HISAMITSU PHARM CO INC
Apparatus and methods for oral administration of fluids and medical instrumentation
ActiveUS20140221917A1Limit ingestionIncrease painOral administration deviceDomestic articlesBuccal administrationIntrathecal
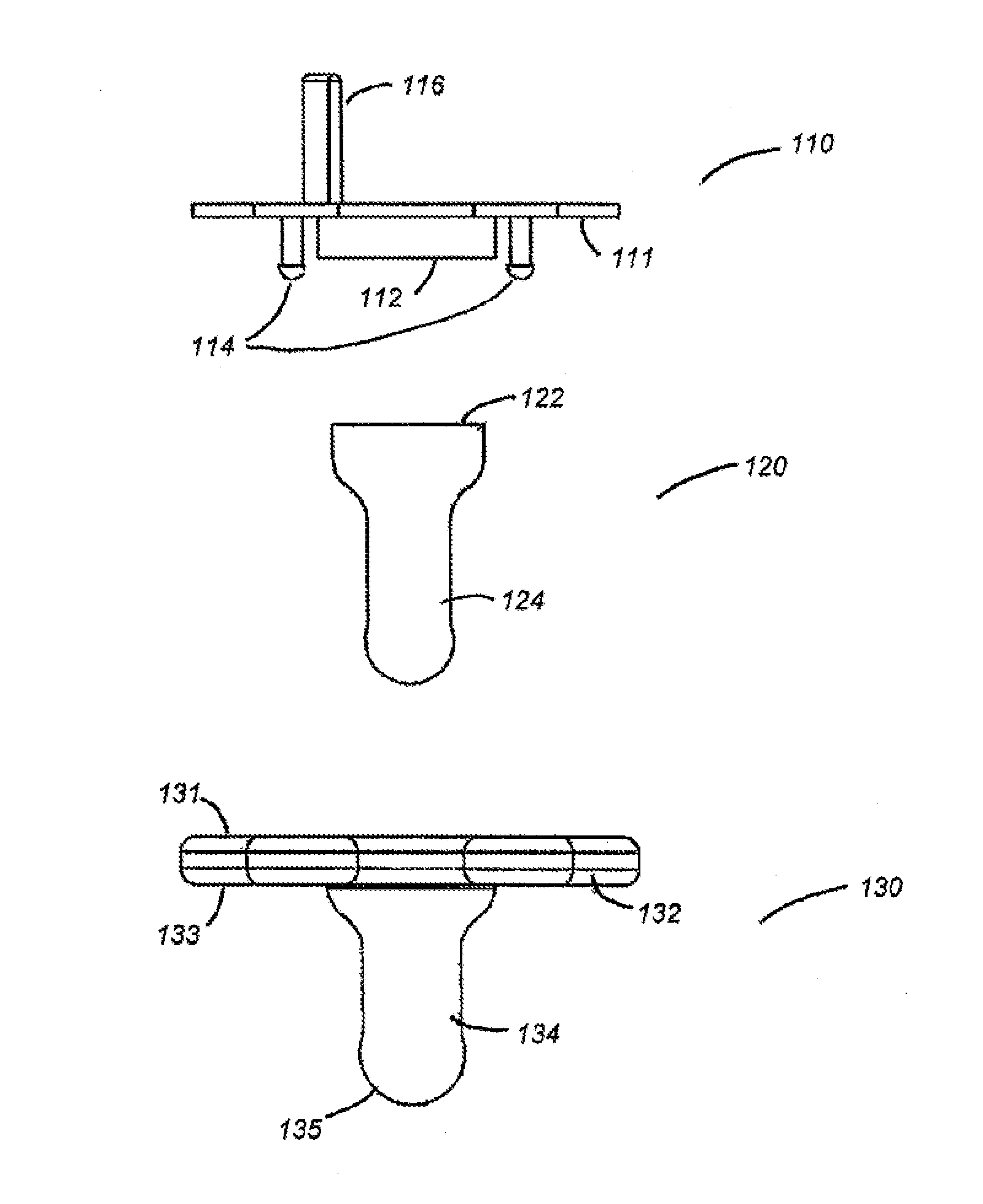
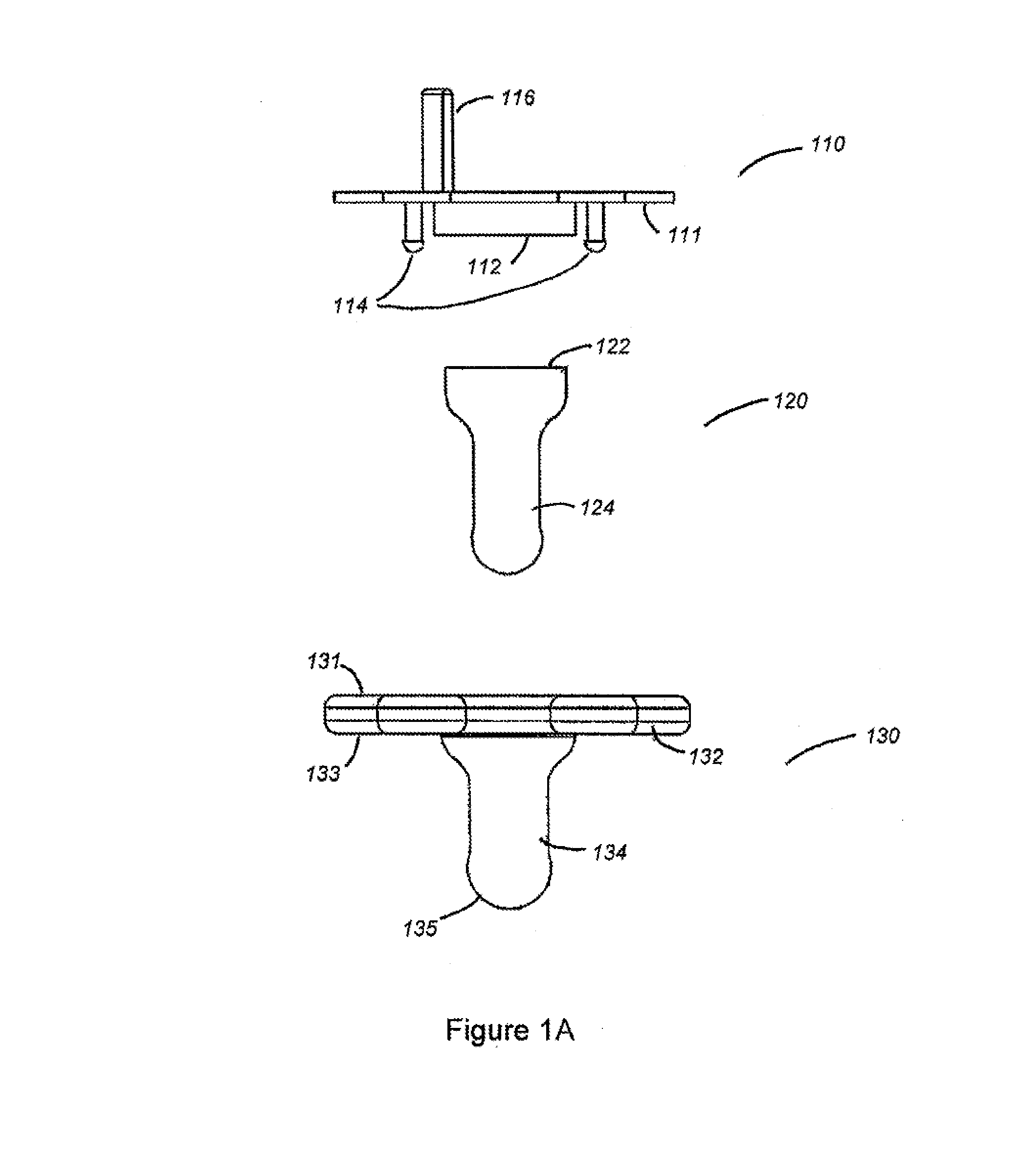
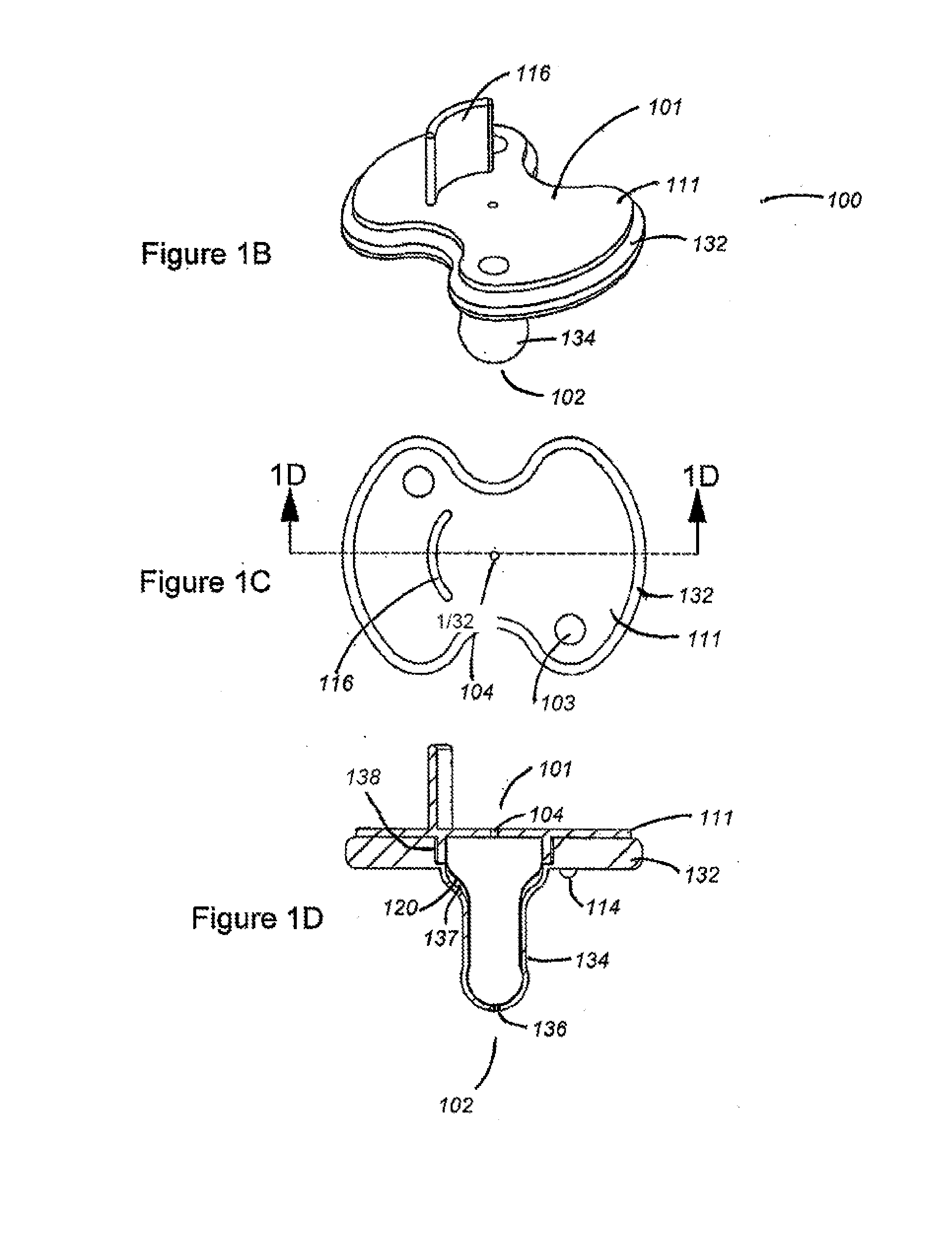
Methods and devices for orally administering fluids and medical instrumentation to individuals for the promotion of health are disclosed. An apparatus is described comprising a pacifier configured to hold fluid and configured for sucking. In some embodiments, the apparatus includes a balloon positioned within a cavity of the pacifier. The balloon is configured to facilitate expulsion of the fluid from the cavity and to limit a user's ingestion of air. In other embodiments, the apparatus includes a cartridge or ampoule configured to store a fluid and expel the fluid through the pacifier. Systems are disclosed which include the pacifier apparatus, a known quantity of fluid or powder, and sterile packaging. Other systems are disclosed whereby various attachments, both medical and non-medical, couple to the apparatus. Methods of using and methods of manufacturing are also disclosed.
Owner:FRIDABABY LLC
Thermosensitive in-situ gel preparation for vaginal administration
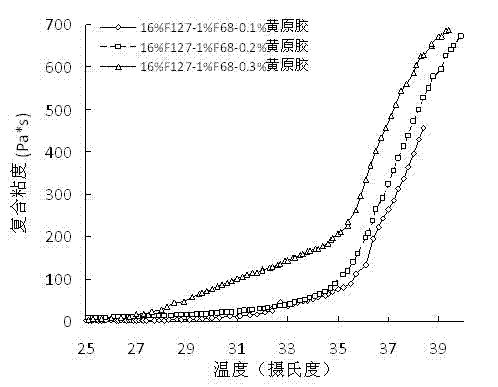
InactiveCN102525884AReverse thermosensitivityEasy to administerAntibacterial agentsAntimycoticsGel preparationIrritation
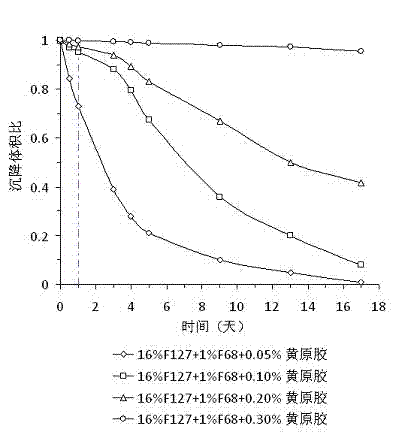
The invention belongs to the field of pharmaceutical preparations and relates to an in-situ gel sustained-release preparation which is used for vaginal administration and has high drug loading and a preparation method of the in-situ gel sustained-release preparation. The preparation is formed by an effective dose of drugs, a thermosensitive gel matrix material, a suspending agent and water or a buffer solution, wherein the drugs are suitable for vaginal administration, the thermosensitive gel matrix material is poloxamer, and the suspending agent is xanthan gum; and the suspending agent, i.e. the xanthan gum, does not affect the thermosensitivity of the preparation and can exert an excellent suspending effect on the drugs. The preparation has the advantages of simplicity in preparation, high drug loading, convenience in administration and little irritation, good safety, wide source of raw materials and low cost.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com