Dosage forms for oral administration and methods of treatment using the same
a technology of effective agents and dosage forms, applied in the direction of drug compositions, enzymology, metabolism disorders, etc., can solve the problems of medical privacy, children's potential stigmatization of children by peers, and subjects often experience difficulty in complying with this administration schedule, etc., to achieve effective and prolonged treatment, easy ingested
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Loading the Resin Particles with Drug
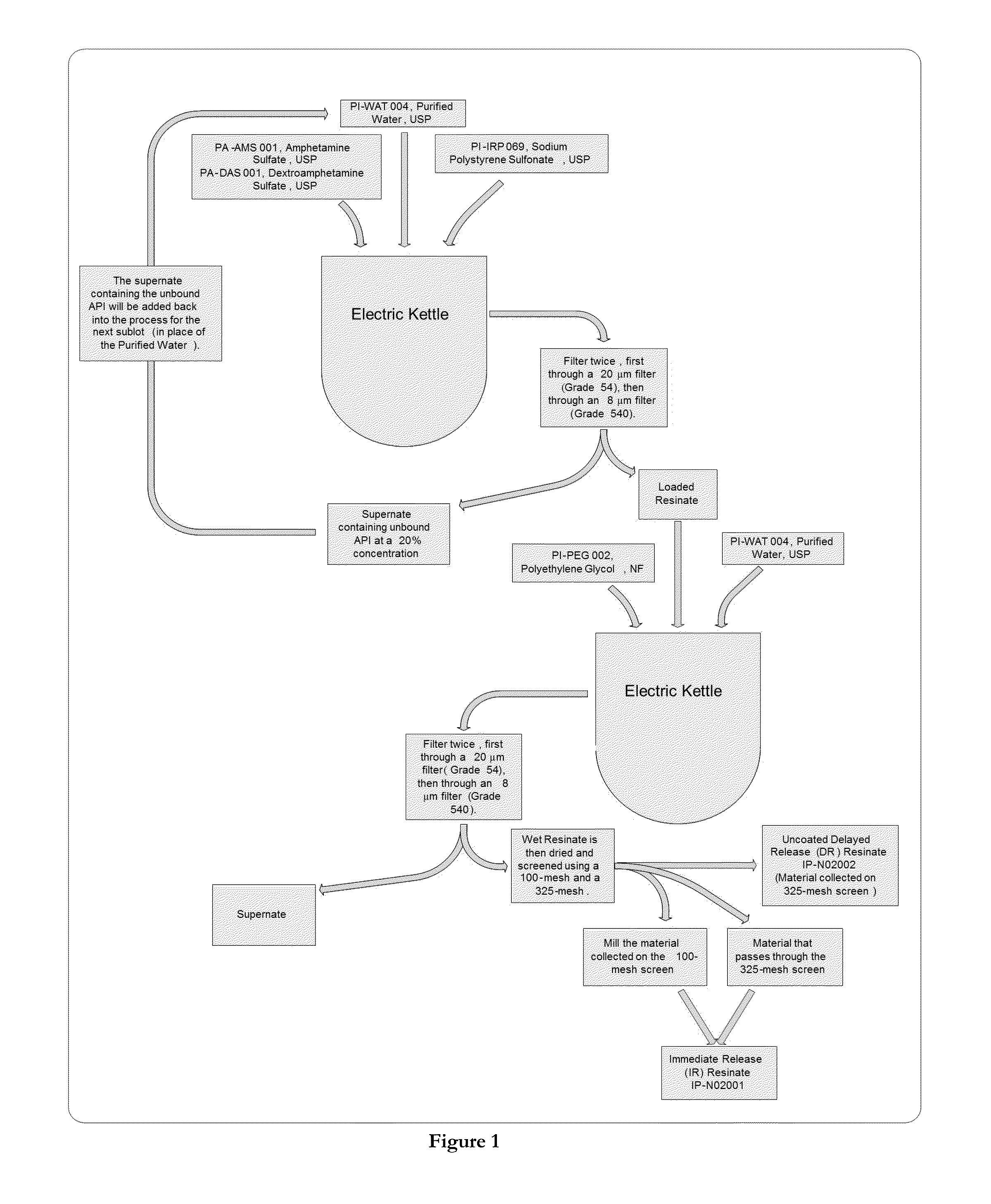
[0168]The invention relates pharmaceutical compositions comprising drug-resin particles and the methods of making these compositions. This example provides an exemplary method of loading amphetamines onto resin particles. FIG. 1 depicts this loading method.
[0169]In a kettle, amphetamine sulfate and dextroamphetamine sulfate are mixed in purified water until fully dissolved. AMBERLITE IRP 69 resins are added to the kettle and mixed. The solution is filtered through a 20 μm filter (Grade 54), then again through a 8 μm filter (Grade 540). The loaded resinate is collected on the filter paper during each filtration.
[0170]In a kettle, polyethylene glycol is mixed in purified water until fully dissolved. After it is completely dissolved, the loaded resinate is added, and the solution is mixed until uniform. The solution is filtered through a 20 μm filter (Grade 54), then again through a 8 μm filter (Grade 540). The loaded resinate / PEG is collected on th...
example 2
Coating Drug-Resin Particles
[0174]The pharmaceutical compositions of the invention may comprise a coated and uncoated plurality of drug-resin particles. The coated drug-resin particles provide the delayed or triggered-release portion of the composition. This example provides an exemplary method of preparing a polymer coating for the coated drug-resin particles.
[0175]A clean, stainless steel container is pre-weighed. Acetone and ethanol are added to the container. Plasticizer is then added to the container and mixed until dispersed. A coating polymer such as HPMC, is slowly added to the container while mixing. The polymer solution is continuously stirred for at least an hour and until all of the solids are dissolved. This coating solution is continuously stirred during the coating process. The loaded resinate of Example 1 may be coated (e.g., in a wurster coater) with this coating solution to prepare coated drug-resin complexes.
example 3
Loading Resin Particles with Drug and Coating the Drug-Resin Particles
[0176]The methods of Examples 1 and 2 may be combined to prepare the pharmaceutical compositions of the invention. In particular, drug-resin particles may be prepared using the method of Example 1, and those drug-resin particles to be used as the delayed release portion of the composition may be coated with a polymer coating prepared by the method of Example 2. The particles may be dried and mixed in a V-blender. The specific ratio of immediate release and delayed release particles may vary as described below.
[0177]Once the resin particles are loaded, coated, and mixed, the resulting drug-resin particles may be used in any suitable dosage form (e.g., suspension, chewable composition, orally disintegrating composition, capsule, tablet, etc.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com