Phenytoin sodium sustained-release suspension and preparation method thereof
A slow-release suspension and phenytoin sodium technology, which is applied in the direction of pharmaceutical formulas, medical preparations with no active ingredients, medical preparations containing active ingredients, etc. To solve the problems such as large fluctuations in blood drug concentration, to achieve the effect of stable drug release, good stability and long duration of drug effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
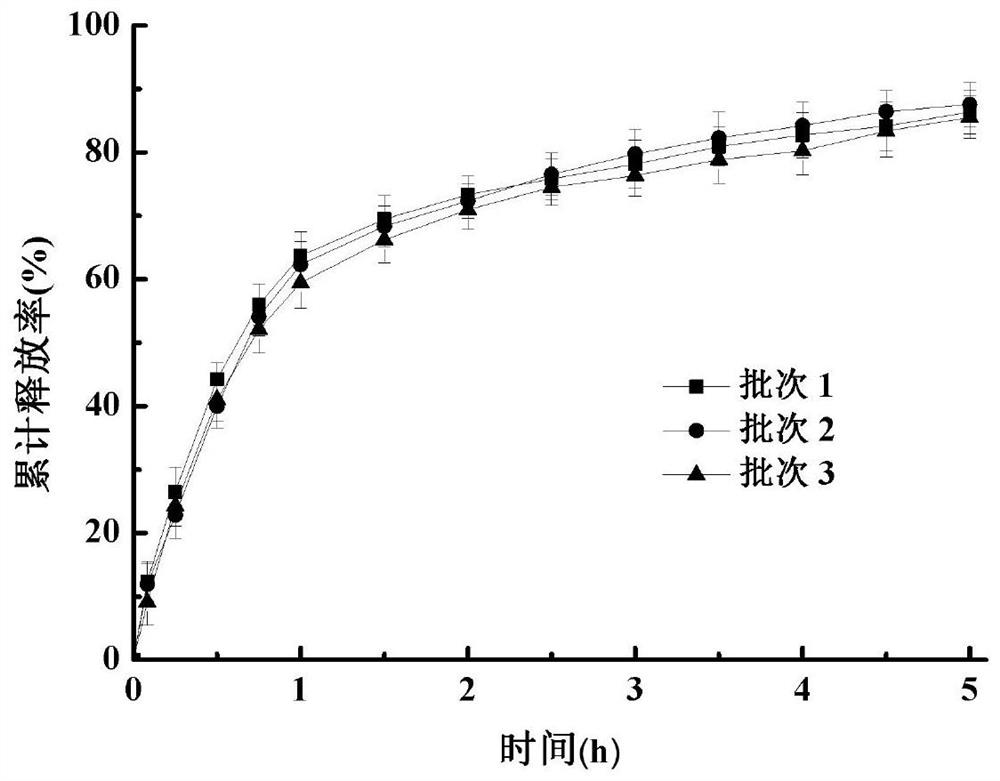
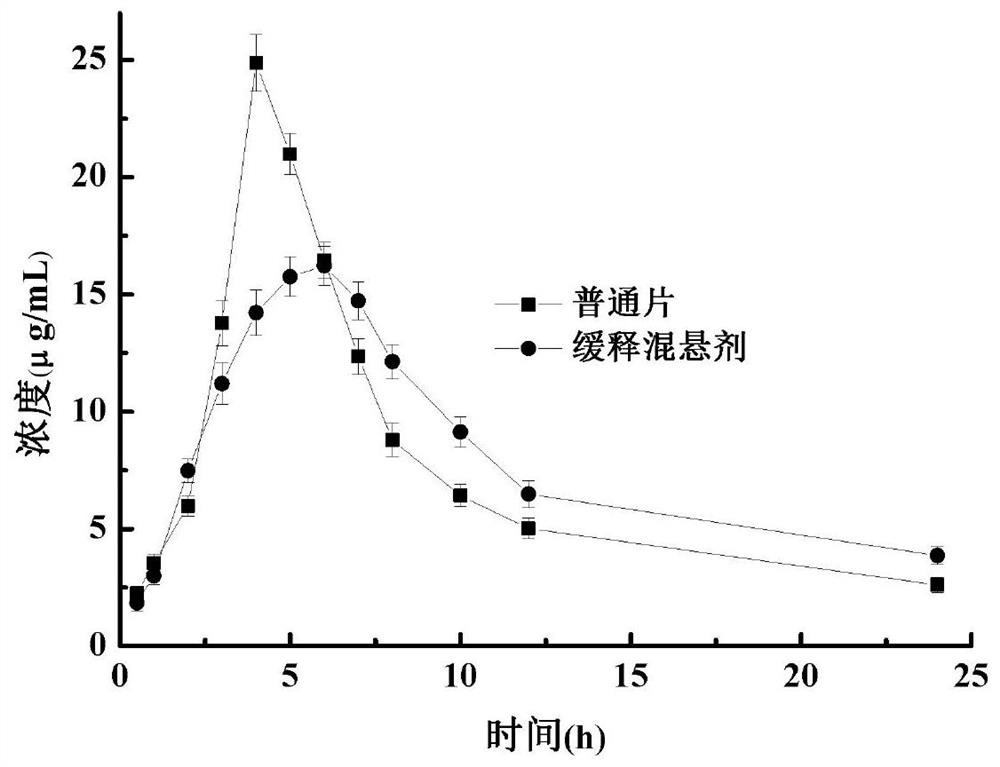
Image
Examples
preparation example Construction
[0021] (1) Preparation of drug resin
[0022] Add the anion exchange resin to deionized water, add the drug under stirring and mix evenly, take samples regularly, and measure the concentration of the drug in the solution; when the drug concentration no longer changes with time, it will reach equilibrium, and wash off the resin surface with deionized water. Unbound drug, dried at 40°C-60°C to obtain drug-loaded resin;
[0023] (2) Impregnation of drug resin
[0024] Take the drug-loaded resin, add it to 20% polyethylene glycol 4000 aqueous solution, stir for 0.5 hours, dry and sieve to obtain the impregnated drug resin;
[0025] (3) Preparation of drug resin microcapsules
[0026] Prepare phenytoin sodium resin microcapsules by emulsification-solvent evaporation method, weigh the coating capsule material and plasticizer, add them to the acetone liquid, stir and dissolve as the dispersed phase; slowly add the impregnated phenytoin sodium resin into the dispersed phase , and s...
Embodiment 1
[0031] Prepare phenytoin sustained-release suspension according to the following formula.
[0032] Phenytoin 50mg
[0033] Anion resin 100mg
[0034] Propylene glycol 10mL
[0035] Macrogol 400 5mg
[0036] Macrogol 4000 50mg
[0037] Acrylic resin (Eudragit RS100, Eudragit RS100) 100mg
[0038] Xanthan Gum 50mg
[0039] Sucrose 10mg
[0040] Propylparaben 3mg
[0041] Banana flavor 15mg.
Embodiment 2
[0043] Prepare phenytoin sustained-release suspension according to the following formula.
[0044] Phenytoin 80mg
[0045] Anion resin 55mg
[0046] Propylene glycol 8mL
[0047] Macrogol 400 1.5mg
[0048] Macrogol 4000: 40mg
[0049] Acrylic resin (Eudragit RS100, Eudragit RS100) 55mg
[0050] Xanthan Gum 40mg
[0051] Povidone (K30) 15mg
[0052] Propylparaben 3mg
[0053] Banana flavor 15mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com