Phenytoin sodium dropping pill and preparation method thereof
A technology of sodium phenytoin and sodium dripping pills, which is applied in the fields of pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc. It can solve problems such as inability to quickly exert drug effects, poor curative effect on arrhythmia, large volume and difficulty in swallowing, etc., to achieve The preparation process is simple and easy, the drug compliance is high, and the production cost is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
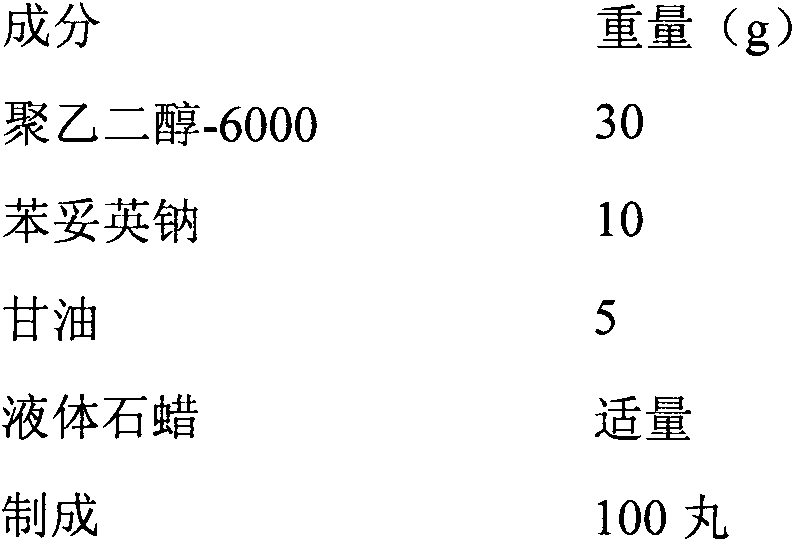
[0016] Each pill contains 100 mg of phenytoin sodium and is prescribed as:
[0017]
[0018] The preparation process is as follows:
[0019] After the polyethylene glycol-6000 was melted at 70°C, sodium phenytoin and glycerin were added, mixed, and stirred for 5 minutes. In ice water, take out and wash after about 10 minutes, place at room temperature, and dry to obtain phenytoin sodium dropping pills.
[0020] Evaluation: The prepared phenytoin sodium dropping pills have bright appearance, bad shape, and are easy to solidify during the dropping process.
Embodiment 2
[0022] Each pill contains 100 mg of phenytoin sodium and is prescribed as:
[0023]
[0024] The preparation process is as follows:
[0025] Same as Example 1.
[0026] Evaluation: The prepared phenytoin sodium dropping pills have bright appearance, bad shape and tailing.
Embodiment 3
[0028] Each pill contains 100 mg of phenytoin sodium and is prescribed as:
[0029]
[0030] The preparation process is as follows:
[0031] After melting polyethylene glycol-6000 at 80°C, add phenytoin sodium, mix and stir for 5 minutes. Under the condition of heat preservation, dropwise into liquid paraffin to form pellets, the drop distance is about 5cm, and then the liquid paraffin is placed in ice water. , about 10 minutes later, take out and wash, place at room temperature, and dry to obtain phenytoin sodium dropping pills.
[0032] Evaluation: The prepared phenytoin sodium dropping pills have bad appearance and tailing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com