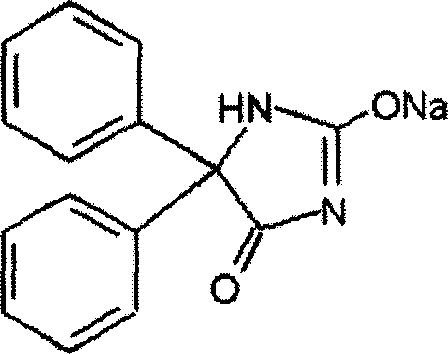
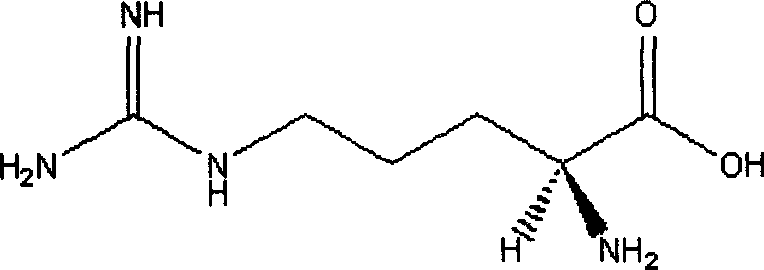
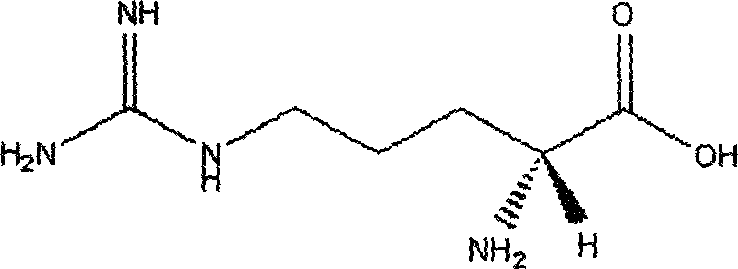
Phenytoin sodium powder injection and preparation method thereof
A technology of sodium phenytoin and powder injection, which is applied in the field of preparation of the powder injection, can solve problems such as difficulty in observing the clarity of intravenous drip infusion, difficulty in draining air bubbles, and turbidity of the solution, so as to increase drug safety and reduce irritation , the effect of increased clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A phenytoin sodium powder injection, the phenytoin sodium powder injection is composed of active ingredient phenytoin sodium and cosolvent arginine. in:
[0037] Arginine 0.26g
[0038] In this embodiment, the arginine is L-arginine or R-arginine.
[0039] The phenytoin sodium powder injection in the present embodiment adopts following steps to prepare:
[0040] 1. Take 0.1g of phenytoin sodium sterile powder and 0.26g of aseptically processed arginine through a 65 mesh sieve;
[0041] 2. Divide 0.1g of phenytoin sterile powder and 0.26g of arginine into sterilized vials, immediately stopper them and seal them with aluminum-plastic caps.
Embodiment 2
[0043] In this example
[0044] Phenytoin Sodium 1000g
[0045] Arginine 2600g
[0046] Others are the same as in Example 1.
[0047] The phenytoin sodium powder injection in this example is prepared using the following steps: 1. Take 1000g of phenytoin sodium sterile powder passing through a 65 mesh sieve and 2600g of aseptically treated arginine passing through a 65 mesh sieve, and put them in SYH-15 Mix evenly in a three-dimensional motion mixer under vacuum conditions; 2. After the uniformity of the sampling inspection is qualified, the mixed powder is divided into 10,000 sterilized vials with a loading capacity of 0.36±0.025g, and the stopper is immediately used and aluminum The plastic cap is sealed. Tested according to the requirements of the Pharmacopoeia, the clarity is qualified.
Embodiment 3
[0049] In this example,
[0050] Phenytoin Sodium 0.25g
[0051]Arginine 0.65g
[0052] Others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com