An alepsin slow-releasing gel for promoting paradontal part reborn as well as preparation method and application
A technology of sodium phenytoin and slow-release gel, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas to achieve stable performance, significant curative effect, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: the preparation method of phenytoin sustained-release gel:
[0060] Raw material: purified water
[0061] 40mg / ml PLGA in dichloromethane
[0062] 80μg / ml phenytoin aqueous solution
[0063] 4% gelatin solution (dissolved in a water bath at 37°C).
[0064] 1.1 W / O / W emulsified solvent evaporation method to prepare PLGA microspheres:
[0065] Mix 100 μl of purified water or an equivalent 80 μg / ml phenytoin sodium solution (inner aqueous phase W 1 ) is added dropwise to the dichloromethane solution (O) of 2ml 40mg / mlPLGA, mixes 1.5min emulsification with the rotating speed of 15000 rev / mins and makes primary emulsion, then it is added dropwise in 10ml 4% gelatin solution (outer aqueous phase W 2 ), mixed at 15000 rpm for 2 minutes to make double emulsion, after 4 hours of magnetic stirring at 8000 rpm at room temperature, centrifuged at 10000 rpm for 10 minutes, removed the sediment in the lower layer, and washed it repeatedly with deionized water for 5 ...
Embodiment 2
[0068] Embodiment 2: The physicochemical performance investigation result of microsphere:
[0069] 2.1 Microsphere particle size and particle size distribution
[0070] 2.1.1 Morphological observation results of microspheres
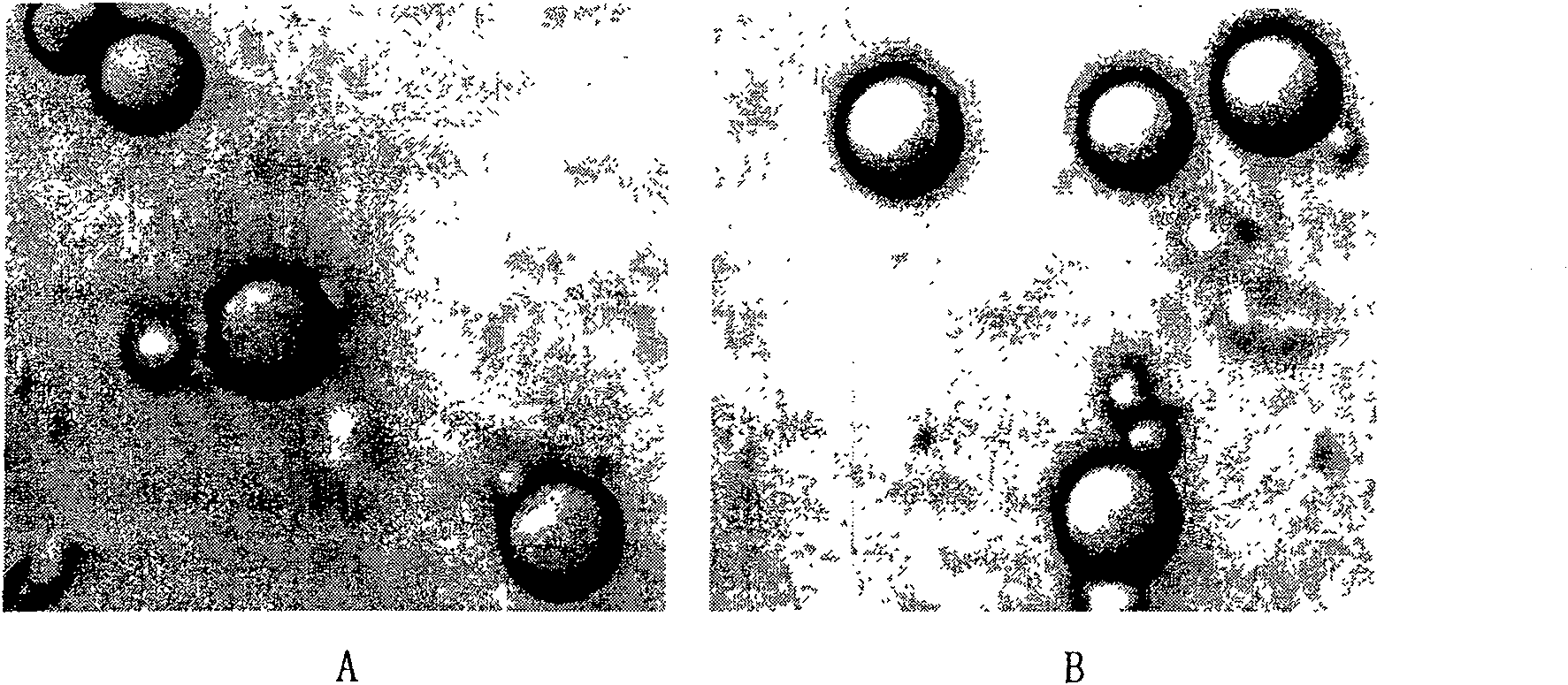
[0071] The shape of the microsphere is a spherical solid particle, the appearance is round, the surface is smooth, and there is no adhesion phenomenon. microscope photo see figure 1 .
[0072] 2.1.2 Examination results of particle size and particle size distribution of microspheres
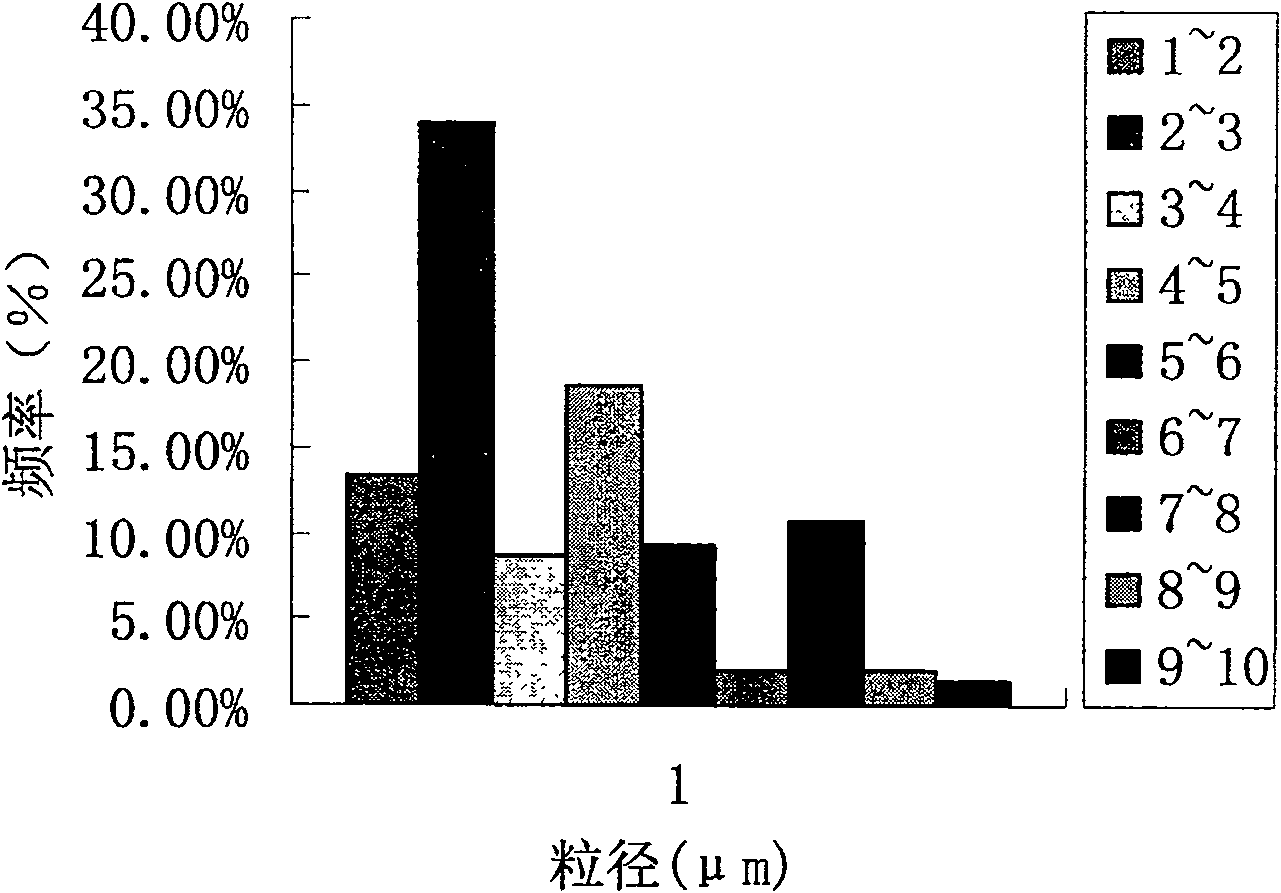
[0073] Count more than 500, according to the formula d av =∑nd / ∑n Calculate the average particle size of the microspheres as (3.93±0.003) μm; take the particle size of the microspheres as the abscissa, divide the particle size range per 1 μm, and within this range, the microspheres The distribution frequency (%) of the microspheres obtained by dividing the number by the total number is the ordinate to draw a particle size distribution diagram, see figure 2 ; Take the...
Embodiment 3
[0097] Example 3: In vivo and local application of phenytoin sodium sustained-release gel to experimental periodontal defects in the molar area of rats and its effect determination:
[0098] Thirty-two adult healthy male Wistar rats (body weight 280-300 g) (provided by the Animal Experiment Center of Shandong University), each animal was randomly divided into two groups with the left mandibular molar area as the experimental site: A, control group (surgically established dental periodontal defect model, implanted with blank microsphere gel); B, experimental group (after surgical establishment of periodontal defect model, implanted with microsphere gel containing phenytoin sodium); 16 animals in each group, of which 8 were randomly selected They were killed 14 days after operation, and the remaining 8 animals were killed 28 days after operation.
[0099] After the rats were anesthetized, they were fixed on the operating table, the skin was prepared in the left mandibular area...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com