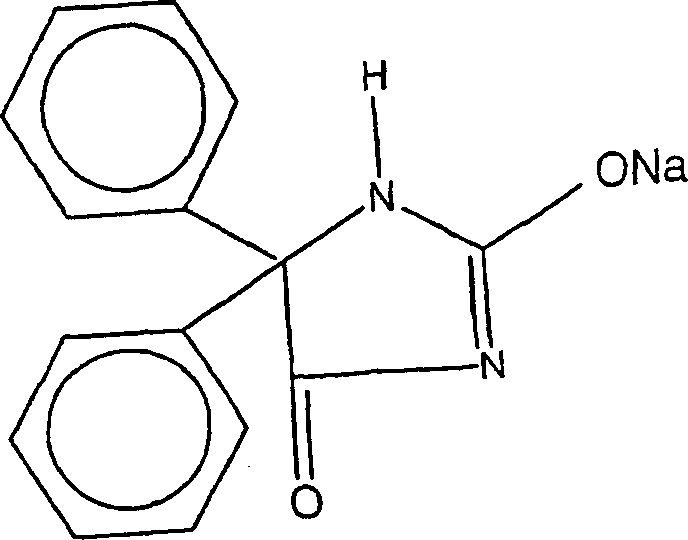
Compaction process for manufacture of sodium phenytoin dosage form
A technology of sodium phenytoin and preparations, which is applied in the field of preparation of dosage forms of sodium phenytoin, which can solve the problems of delayed absorption and inability to change drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The blends of phenytoin and excipients were provided in the amounts shown in Table 1. The mixture was blended in the Patterson-Kelly(R) for 10 minutes.
[0046] Composition % (total weight)
Embodiment 2
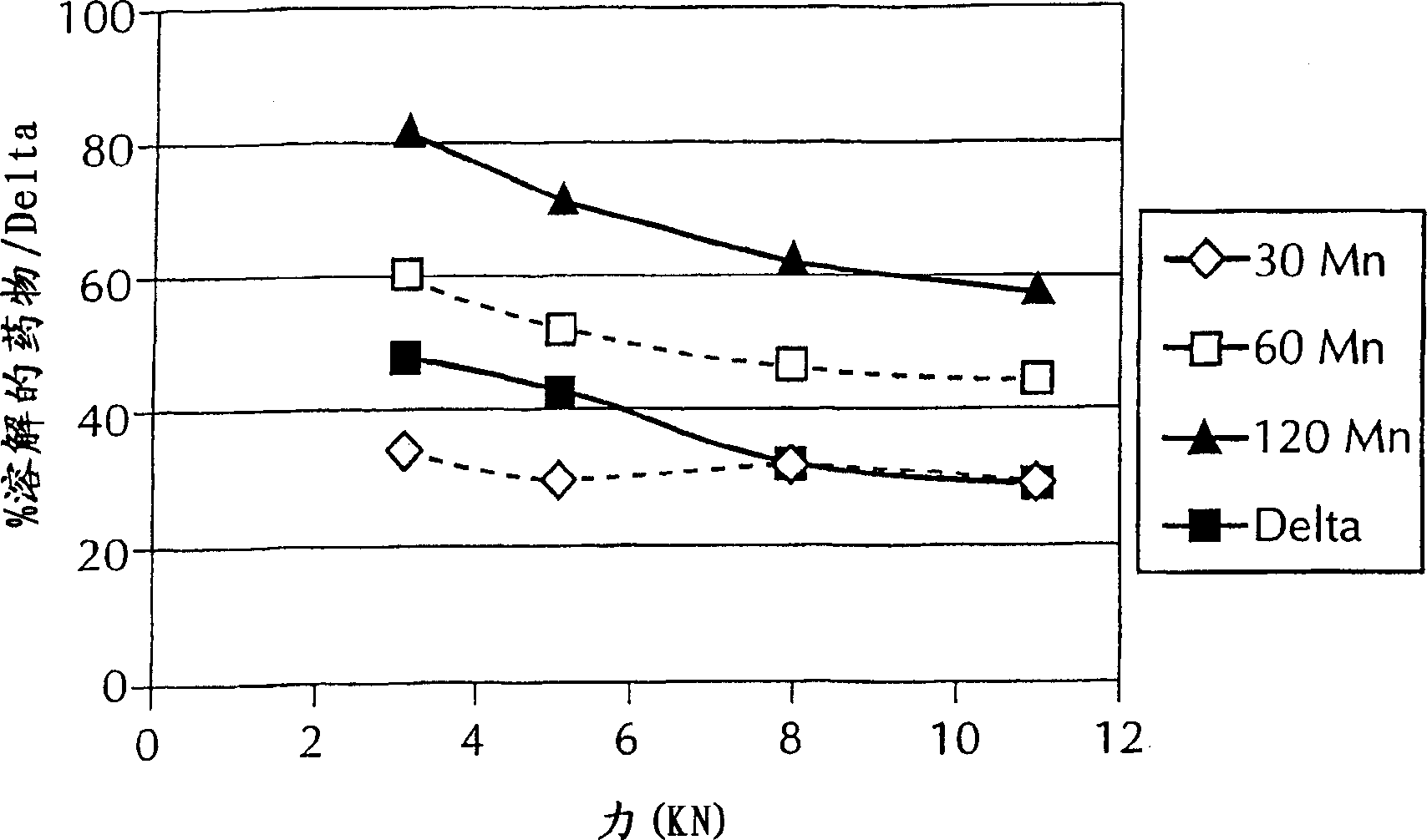
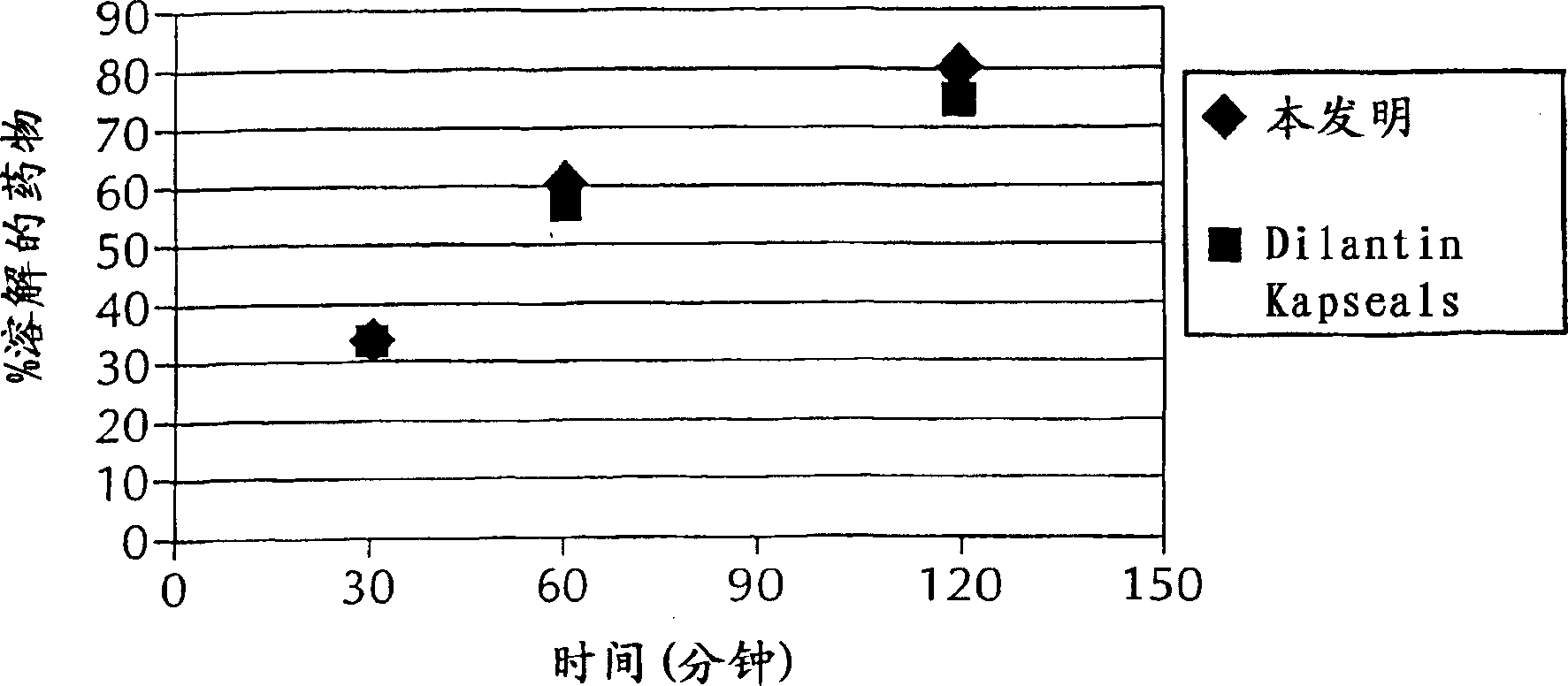
[0048] To determine the extent to which compressive forces play a role in the dissolution of granules produced by the process of the present invention, the roller gap and speed process parameters were held constant as described below. Table 2 provides the dissolution data for portions of the blend described in Example 1 compressed at various roller pressures. The percentage of drug dissolved is determined using standard methods well known in the art. Specifically, each phenytoin formulation was tested using the USP dissolution test. Specifically, the test involved placing each capsule in 900 mL of water maintained at a temperature of 37°C ± 0.5°C and stirred at 50 rpm. Samples were taken at 30, 60 and 120 minutes and the amount of dissolved phenytoin was determined.
[0049] Table 2. Effect of compression force on dissolution
[0050] Method Parameters: Dissolved (%)
[0051] Roll gap = 2mm (sd)
[0052] Roll speed = 3rpm, n = 12
[0053] Roller pressure 30 minutes 60 mi...
Embodiment 3
[0062] To determine the extent to which compression force alone affects dissolution, all processing parameters except roller pressure were held constant as shown in Table 2 above. However, Table 3 provides dissolution data for various blend samples described in Example 1 at different roller pressures, roller gap widths (distance between the outer edges of the rollers at their closest point) and roller speeds. Similar to Example 2, the percentage of drug dissolved was determined using standard methods well known in the art.
[0063] Table 3. Influence of Processing Parameters
[0064] Processing parameters Dissolution (%)
[0065] (sd)
[0066] n=12
[0067] Batch Roller Roller Roller 30 minutes 60 minutes 120 minutes
[0068] Gap Velocity Pressure
[0069] (MM) (RPM) (KN)
[0070] 1 2.5 6.0 7.0 29(2.0) 49(3.2) 66(4.4)
[0071] 2 2.0 3.0 3.0 33(2.9) 62(57) 81(4.7)
[0072] 3 2.5 6.0 11.0 27(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com