Indapamide tablet and preparation method thereof
A technology of indapamide tablets and indapamide, which can be applied to pharmaceutical formulations, medical preparations containing active ingredients, sugar-coated pills, etc., and can solve problems such as the dissolution rate of indapamide tablets is too fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A kind of indapamide tablet, is made up of tablet core and coating layer, and described tablet core is made up of following components:
[0031] 1 part of indapamide, 20 parts of lactose, 5 parts of corn starch, 2 parts of hypromellose, 1 part of talc, 1 part of magnesium stearate;
[0032] The preparation method of above-mentioned indapamide sheet mainly comprises the following steps:
[0033] 1) Pretreatment: crush the indapamide raw material to 50-75 μm, and pass through a 230-mesh sieve through a swing granulator; the excipients are dispersed through a 40-mesh sieve, and set aside;
[0034] 2) Premixing: Weigh the indapamide, lactose, cornstarch, and hypromellose in the above-mentioned preparation amount, put them in a wet mixing granulator, and mix for 10 minutes;
[0035] 3) Granulation: Add wetting agent under the parameters of stirring 3r / s and atomization pressure 0.3 Mpa; continue stirring for 3r / s, and granulate under the parameters of shearing 30r / s; Granu...
Embodiment 2
[0041] A kind of indapamide tablet, is made up of tablet core and coating layer, and described tablet core is made up of following components:
[0042] 1 part of indapamide, 25 parts of lactose monohydrate, 8 parts of corn starch, 1 part of povidone K30, 2 parts of talcum powder, 1 part of silicon dioxide;
[0043] The above-mentioned indapamide bulk drug was crushed to 75-100 μm, and passed through a 150-mesh sieve through a swing granulator; other excipients were dispersed through a 200-mesh sieve, and set aside; other preparation methods were the same as in Example 1.
Embodiment 3
[0045] A kind of indapamide tablet, is made up of tablet core and coating layer, and described tablet core is made up of following components:
[0046] 1 part of indapamide, 23 parts of lactose monohydrate, 6 parts of corn starch, 1 part of povidone K30, 2 parts of talcum powder, 1 part of magnesium stearate;
[0047] The above-mentioned indapamide bulk drug was pulverized to 100-125 μm, and passed through a 120-mesh sieve through a swing granulator; other excipients were dispersed through a 200-mesh sieve, and set aside; other preparation methods were the same as in Example 1.
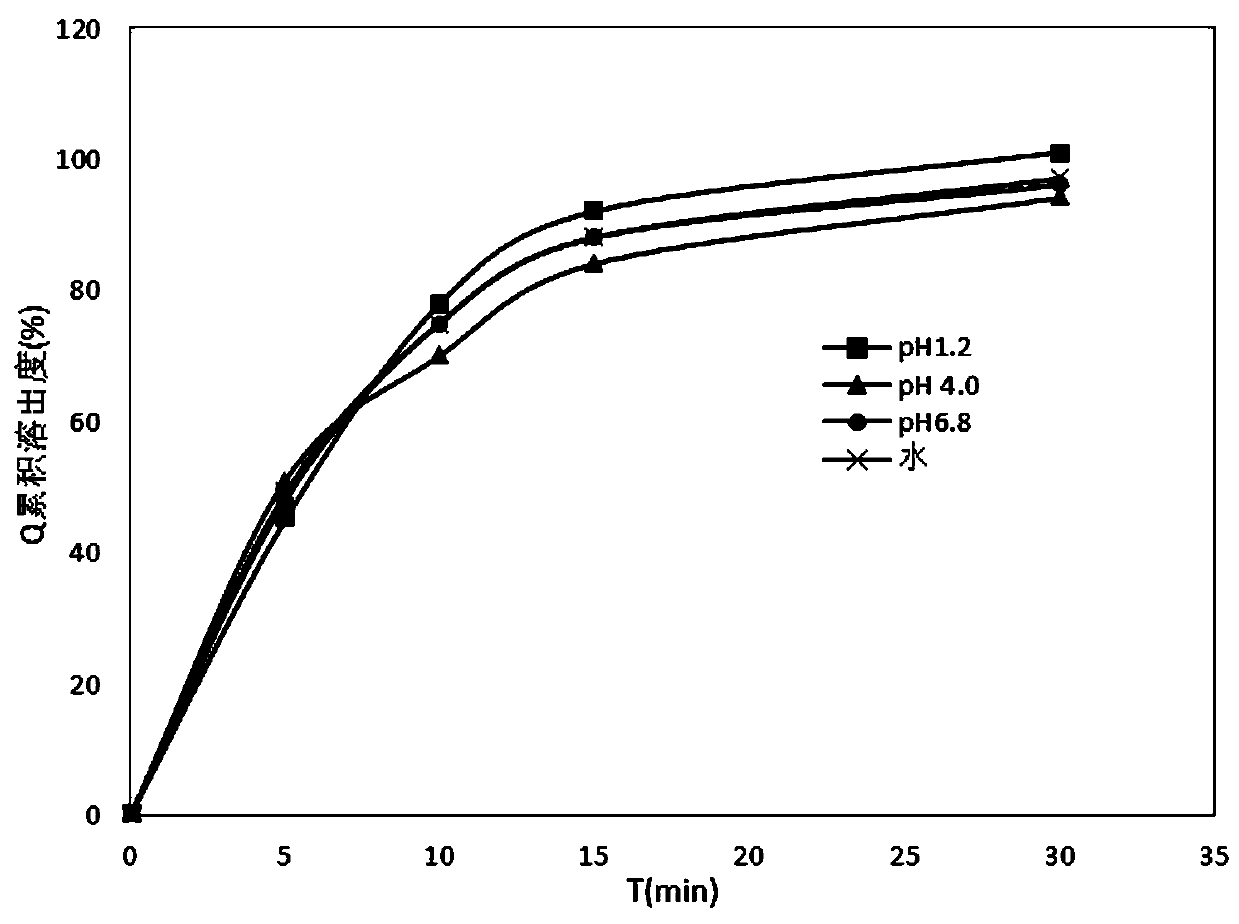
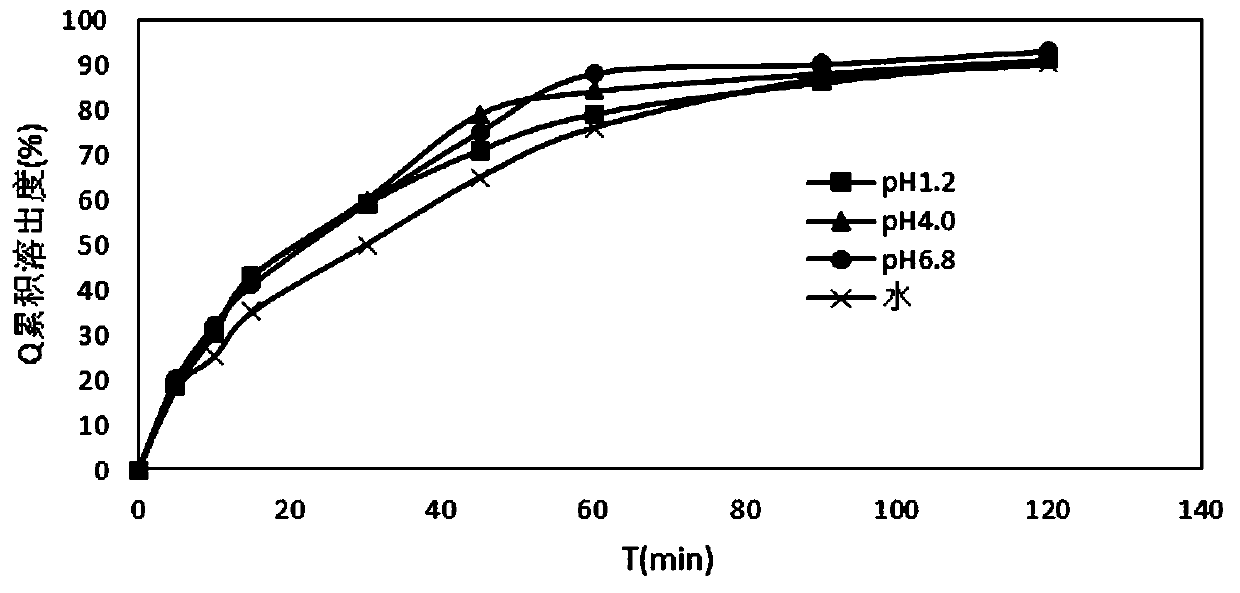
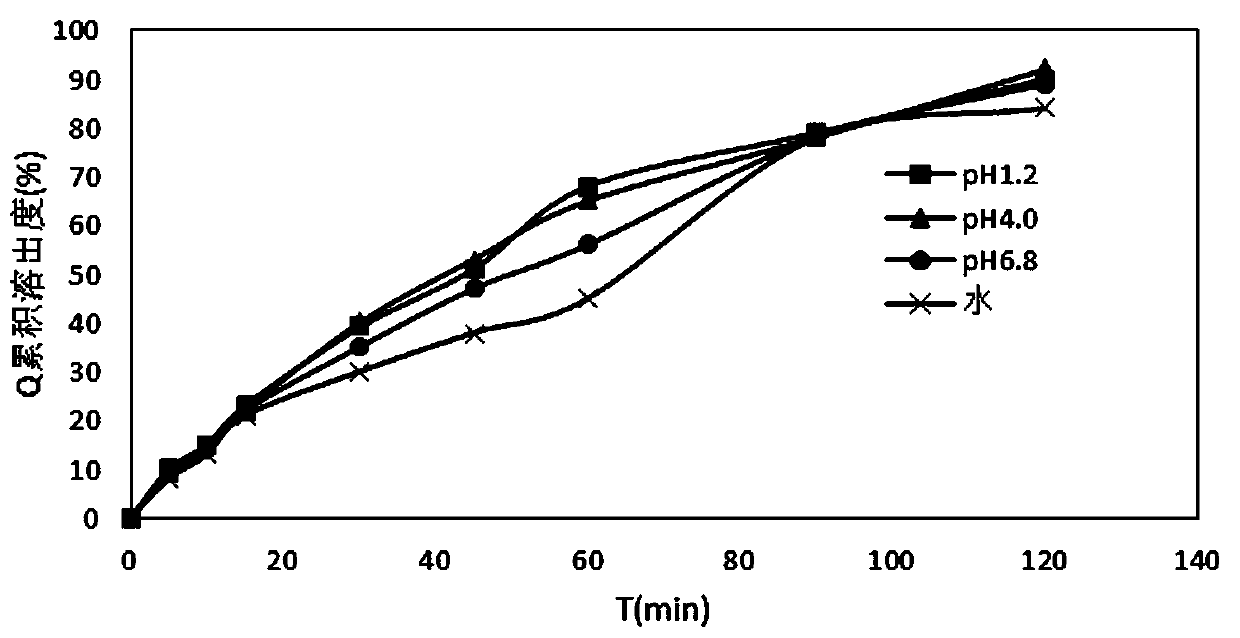
[0048] The properties of the indapamide tablets prepared in the above examples were measured below.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com