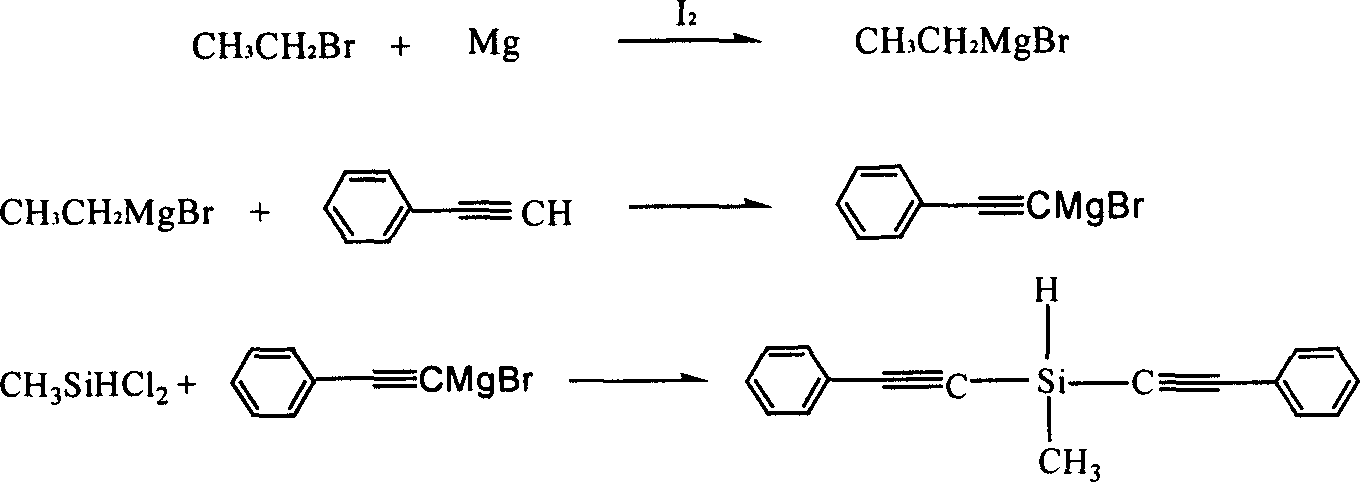
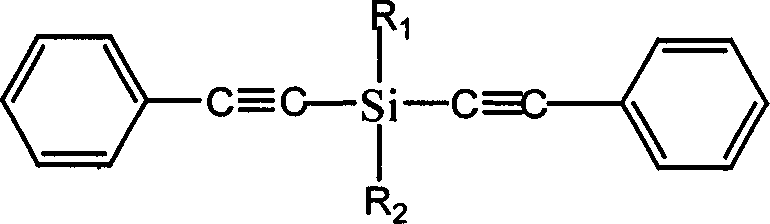
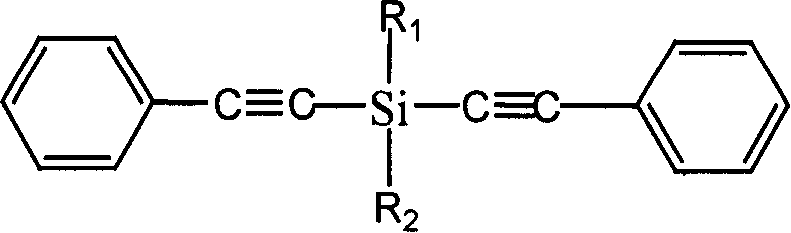Diphenylacetylene silane novle synthesis method
A technology of phenylethynyl and phenylacetylene, which is applied in the field of new synthesis of organosilicon monomers, can solve the problems of only 82% purity, long reaction process, cumbersome process, etc., and achieve easy control of reaction conditions, short reaction time, and process flow simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: 19 mmol of phenylacetylene was dissolved in 10 ml of tetrahydrofuran, and 16 mmol of butyllithium reagent was added dropwise with a constant pressure funnel for 40 minutes, and reacted at -20°C for 5 hours after the addition was completed. Then, 6 mmol of methyldichlorosilane was dissolved in 10 ml of tetrahydrofuran and added dropwise to the phenylethynyllithium reagent, and reacted at 50° C. for 6 hours after the dropwise addition was completed. After the reaction, pour 30ml of ice saturated ammonium chloride solution into the reaction solution, stir fully, separate the liquids, take the upper oil phase, and then wash with 30ml of saturated ammonium chloride solution until the pH value is neutral, and add diethyl ether to the obtained water phase Extraction, liquid separation, collecting and merging the obtained oil phases, and distilling off the solvent under reduced pressure to obtain the final product methyl tolanyl silane.
Embodiment 2
[0026] Example 2: Dissolve 30mmol of phenylacetylene in 15ml of tetrahydrofuran, add 30mmol of butyllithium reagent dropwise with a constant pressure funnel, dropwise for 40 minutes, and react at -10°C for 4 hours after the dropwise addition. Then 12 mmol of methyldichlorosilane was dissolved in 10 ml of tetrahydrofuran and added dropwise to the phenylethynyllithium reagent, and reacted at 40° C. for 6 hours after the dropwise addition was completed. After the reaction is completed, pour 30ml of ice saturated ammonium chloride solution into the reaction solution, stir fully, separate the liquids, take the upper oil phase and wash it once with 30ml of saturated ammonium chloride solution until the pH value is neutral, and the water phase obtained twice Diethyl ether was added for extraction, the layers were separated, the combined oil phases were collected, and the solvent was distilled off under reduced pressure to obtain the final product, methyl tolanyl silane.
Embodiment 3
[0027] Example 3: 22 mmol of phenylacetylene was dissolved in 10 ml of tetrahydrofuran, and 20 mmol of butyllithium reagent was added dropwise with a constant pressure funnel. The addition time was 30 minutes, and the reaction was carried out at -20°C for 5 hours after the addition was completed. Then, 10 mmol of methyldichlorosilane was dissolved in 10 ml of tetrahydrofuran and added dropwise to the phenylethynyllithium reagent, and reacted at 60° C. for 4 hours after the dropwise addition was completed. After the reaction is completed, pour 30ml of ice saturated ammonium chloride solution into the reaction solution, stir fully, separate the liquids, take the upper oil phase and wash it once with 30ml of saturated ammonium chloride solution until the pH value is neutral, and the water phase obtained twice Diethyl ether was added for extraction, the layers were separated, the combined oil phases were collected, and the solvent was distilled off under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com