Bicyclohexylethylene substituted diphenylne liquid crystal compound and preparation method thereof
A technology of liquid crystal compounds and ethylene groups, applied in the field of materials, can solve problems such as high melting point, poor compatibility, and large light absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
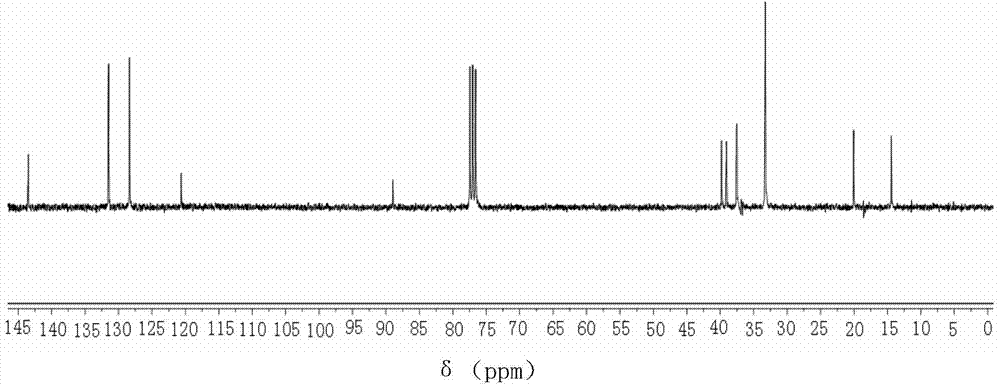
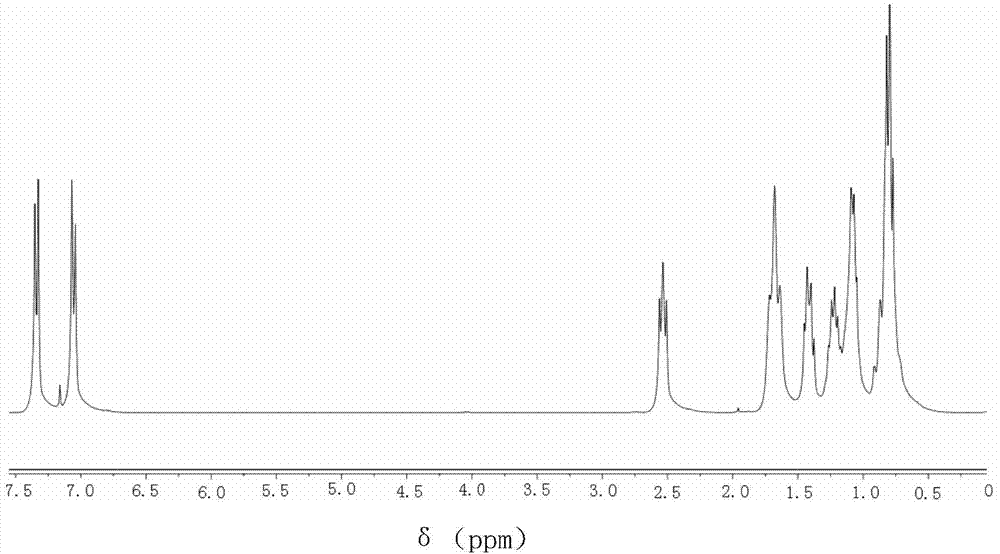
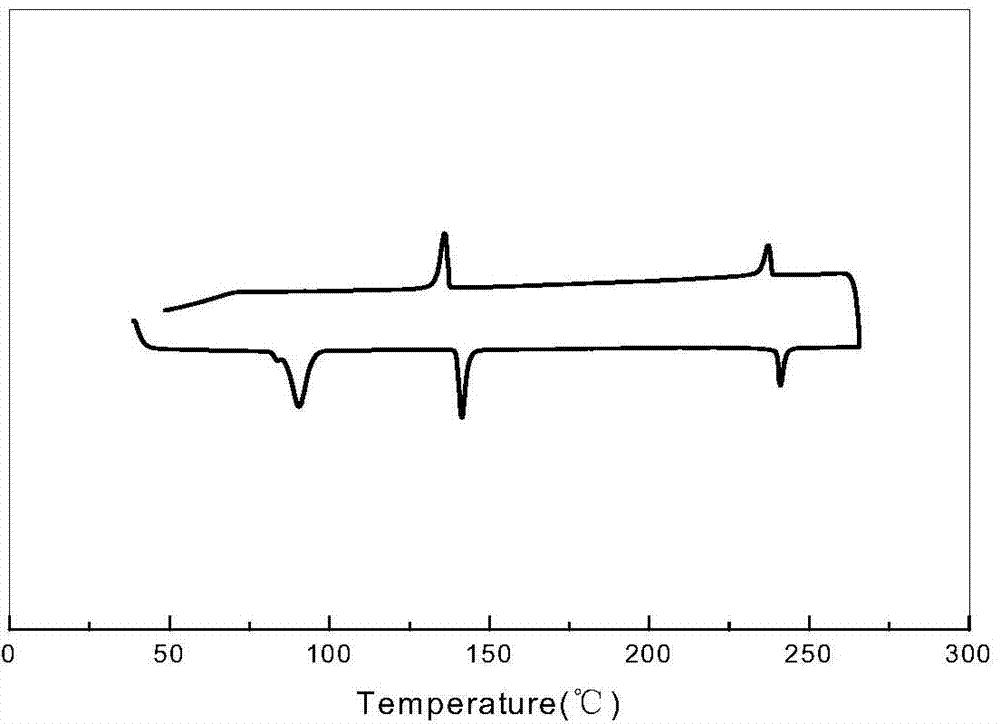
[0069] This example is a liquid crystal compound substituted with biscyclohexylethylene group, which is 1-{4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2-{4- [2-(4-n-propylcyclohexyl)ethyl]phenyl}acetylene, wherein R and R' are both C3 linear alkyl groups, and the values of m, n, and x are all 0.
[0070] The preparation of 1-{4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2-{4-[2-(4-n-propylcyclohexyl)ethyl] proposed in this example The specific process of phenyl}acetylene is:
[0071] Step 1: Synthesis of substituted cyclohexyl formamide: the substituted cyclohexyl formamide described in this example is 4-n-propylcyclohexyl formamide.
[0072]
[0073] Add thionyl chloride and substituted cyclohexyl formic acid in a molar ratio of 1.5:1, and an appropriate amount of toluene into a 1L three-necked flask equipped with a stirrer, a condenser, a thermometer, and a tail gas recovery device. In this example, the added trans-4-n-propylcyclohexylcarboxylic acid is 340.00g, the added th...
Embodiment 2
[0101] This example is a liquid crystal compound substituted with biscyclohexylethylene group, which is 1-{2-fluoro-4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2 -{4-[2-(4-n-pentylcyclohexyl)ethyl]phenyl}acetylene, wherein R is a C3 straight-chain alkyl group, R' is a C5 straight-chain alkyl group, and the value of m is 1 , the values of n and x are both 0.
[0102] The preparation of 1-{2-fluoro-4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2-{4-[2-(4-n-pentylcyclohexyl) ) The specific process of ethyl] phenyl} acetylene is:
[0103] Step 1: Synthesis of substituted cyclohexyl formamide: the substituted cyclohexyl formamide described in this example is 4-n-pentylcyclohexyl formamide.
[0104]
[0105] Replace the trans-4-n-propylcyclohexyl formic acid in step 1 of embodiment one with trans-4-n-pentyl cyclohexyl formic acid of equimolarity, adopt the synthesis process identical with step 1 in embodiment one to synthesize 4-normal Pentylcyclohexylcarboxamide.
[0106] Step 2: Sy...
Embodiment 3
[0142] This example is a liquid crystal compound substituted with biscyclohexylethylene group, which is 1-{2-fluoro-4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2 -{2-fluoro-4-[2-(4-n-pentylcyclohexyl)ethyl]phenyl}acetylene, wherein R is a straight-chain alkyl group of C3, R' is a straight-chain alkyl group of C5, m and The value of n is 1, and the value of x is 0.
[0143] The preparation of 1-{2-fluoro-4-[2-(4-n-propylcyclohexyl)ethyl]phenyl}-2-{2-fluoro-4-[2-(4-n- The specific process of pentylcyclohexyl)ethyl]phenyl}acetylene is:
[0144] Step 1: Synthesis of substituted cyclohexyl formamide: the substituted cyclohexyl formamide described in this example is 4-n-propyl cyclohexyl formamide and 4-n-pentyl cyclohexyl formamide, using the same method as in Example 1 Synthesis of 4-n-propylcyclohexylcarboxamide in the same synthesis process as step 1, and synthesis of 4-n-pentylcyclohexylcarboxamide in the same synthesis process as step 1 in Example 2.
[0145] Step 2: Synthesis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| isotropization temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com