Diphenylacetylene butene liquid crystal compound and synthesis method thereof
A technology of toluene butylene and liquid crystal compound, which is applied in the field of tolan butylene liquid crystal compound and its synthesis, can solve the problems of high melting point and high viscosity, and achieve low melting point, good low temperature performance, high product yield and The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
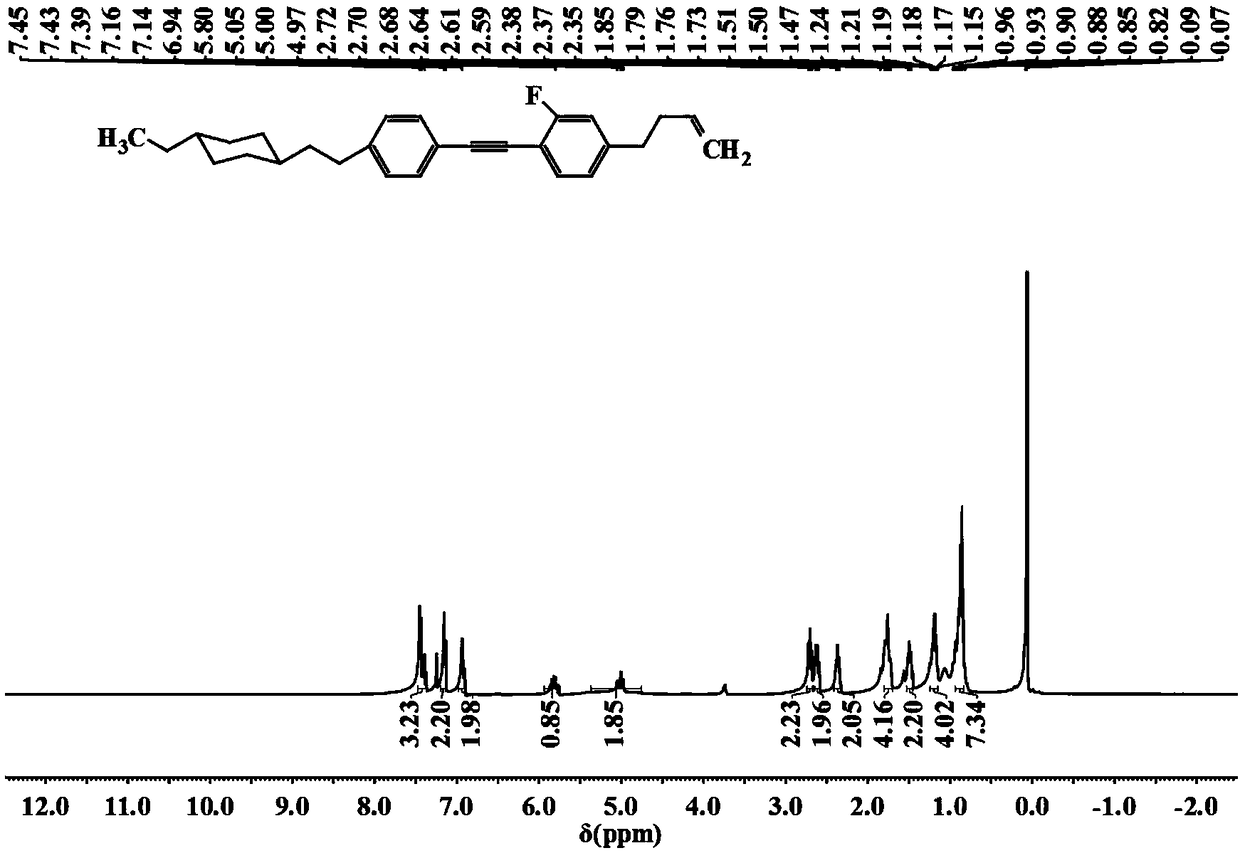
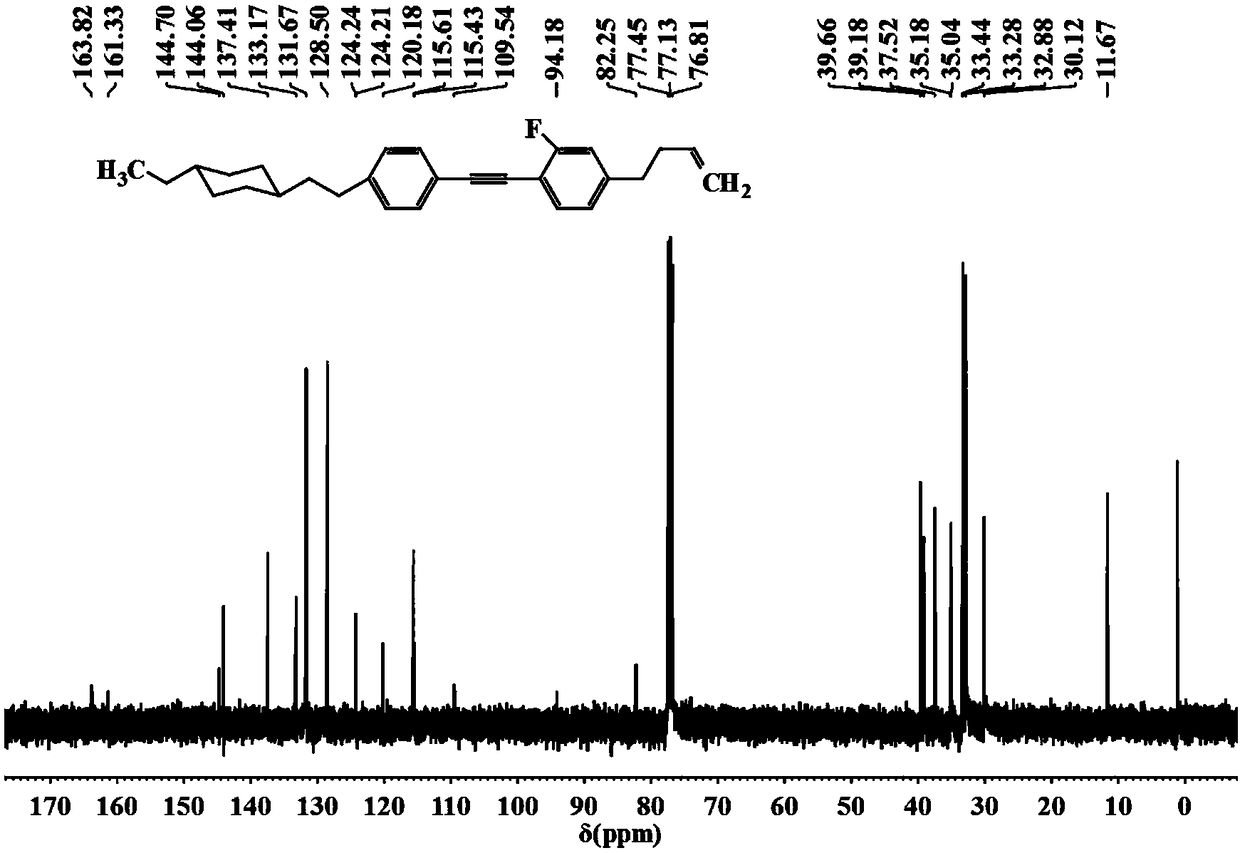
[0034] 1-{2-[4-(but-3-enyl)phenyl]ethynyl}-4-[2-(4-propylcyclohexyl)ethyl]benzene of the following synthetic structure
[0035]
[0036] 1. Under the protection of nitrogen, add 1.29g (3mmol) 2-{4-{2-{4-[2-(4-propyl Cyclohexyl)ethyl]phenyl}ethynyl}phenethyl}-1,3-dioxolane, 50mL tetrahydrofuran, 27.6g (0.6mol) formic acid, heated to 55°C, stirred at constant temperature for 5 hours, cooled to room temperature Dilute with water, extract with dichloromethane, wash the organic phase with water until neutral, dry with anhydrous magnesium sulfate, filter, evaporate the solvent, concentrate, recrystallize the solid with ethyl acetate, and dry to obtain 0.9g of yellow solid 3-{4 -{2-{4-[2-(4-Propylcyclohexyl)ethyl]phenyl}ethynyl}phenyl}propanal, gas chromatography purity 98%, yield 80.8%.
[0037] 2. Under the protection of nitrogen, add 0.82g (2.3mmol) methyl bromide triphenylphosphine salt and 50mL tetrahydrofuran successively to a 100mL three-neck flask equipped with a condense...
Embodiment 2
[0042] Synthesis of 1-{2-[4-(but-3-enyl)-2-fluorophenyl]ethynyl}-4-[2-(4-ethylcyclohexyl)ethyl]benzene with the following structural formula
[0043]
[0044]1. Under the protection of nitrogen, add 0.86g (2mmol) 2-(3-fluoro-4-(2-(4-(2-( 4-Ethylcyclohexyl)ethyl)phenyl)ethynyl)phenethyl)-1,3-dioxolane, 50mL tetrahydrofuran, 18.4g (0.4mol) formic acid, heated to 55°C, stirred at constant temperature for 5 hours , cooled to room temperature, diluted with water, extracted with dichloromethane, washed the organic phase with water until neutral, dried with anhydrous magnesium sulfate, filtered, evaporated to remove solvent, concentrated, solid recrystallized with ethyl acetate, dried to obtain 0.6g yellow solid 3-{4-{2-{4-[2-(4-Ethylcyclohexyl)ethyl]phenyl}ethynyl}-3-fluorophenyl}propanal, gas chromatography purity 98%, yield 80 %.
[0045] 2. Under the protection of nitrogen, add 0.5g (1.4mmol) methyl bromide triphenylphosphine salt and 50mL tetrahydrofuran successively into a...
Embodiment 3
[0050] Synthesis of 1-{2-[4-(but-3-enyl)-2-fluorophenyl]ethynyl}-4-[2-(4-propylcyclohexyl)ethyl]benzene with the following structural formula
[0051]
[0052] 1. Under the protection of nitrogen, add 0.67g (1.5mmol) 2-(3-fluoro-4-(2-(4-(2- (4-Propylcyclohexyl)ethyl)phenyl)ethynyl)phenethyl)-1,3-dioxolane, 50mL tetrahydrofuran, 13.9g (0.3mol) formic acid, heated to 55°C, stirred at constant temperature for 5 hours, cooled to room temperature, diluted with water, extracted with dichloromethane, washed the organic phase with water until neutral, dried with anhydrous magnesium sulfate, filtered, evaporated to remove solvent, concentrated, solid recrystallized with ethyl acetate, dried to obtain 0.5g yellow Solid 3-{4-{2-{4-[2-(4-propylcyclohexyl)ethyl]phenyl}ethynyl}-3-fluorophenyl}propanal, gas chromatography purity 98%, yield 83%.
[0053] 2. Under the protection of nitrogen, add 0.4g (1.1mmol) methyl bromide triphenylphosphine salt and 50mL tetrahydrofuran successively in...
PUM
| Property | Measurement | Unit |
|---|---|---|
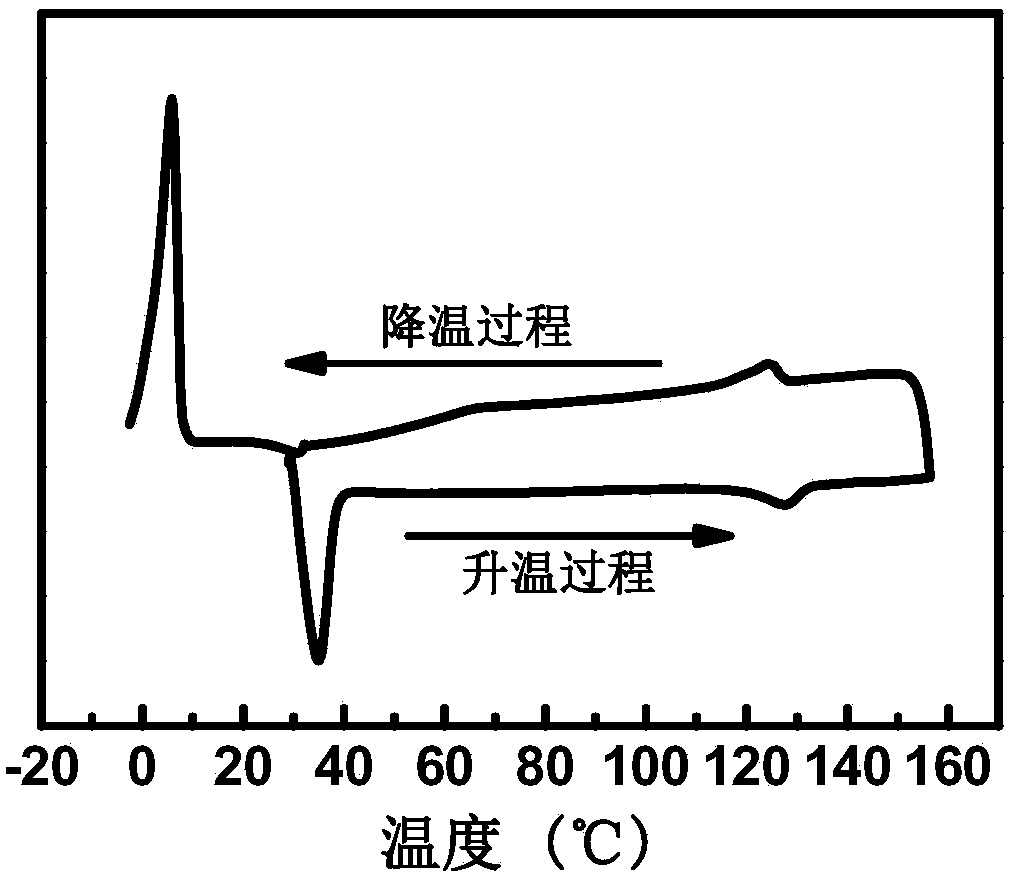
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com