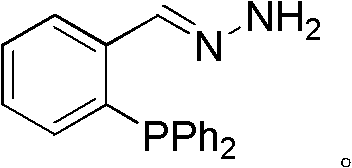
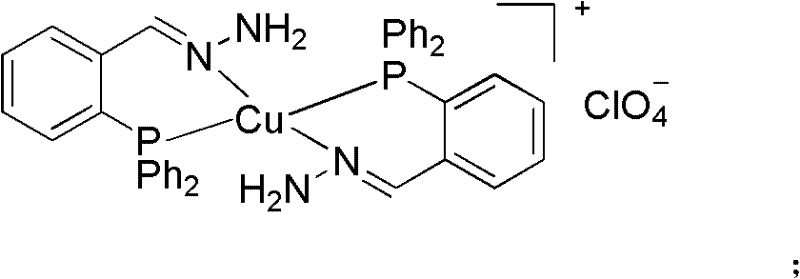
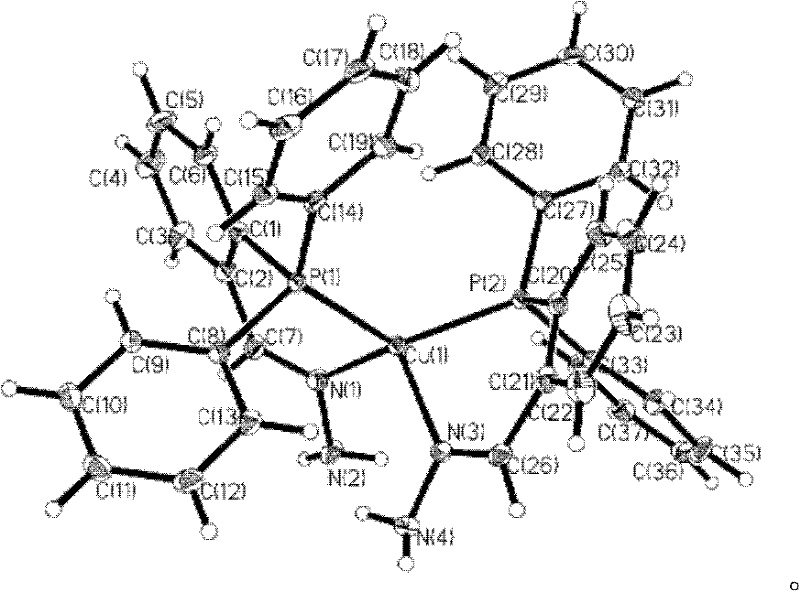
Phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine as well as synthetic method and application of phosphine imide ligand (E)-(2-(diphenyl phosphino) benzylidene) hydrazine
A technology of diphenylphosphino and benzylidene, applied in the field of catalyst ligand preparation, can solve the problems of unsatisfactory results, unsatisfactory ligand activity, toxic palladium and the like, and achieves short reaction time , The effect of convenient post-processing and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] A kind of synthetic method of described phosphine imide ligand (E)-(2-(diphenylphosphino)benzylidene)hydrazine, the steps are as follows:
[0026] (1) Preparation of intermediate 2-diphenylphosphinobenzaldehyde
[0027] 1) Add 25.0g (0.131mol) o-bromobenzaldehyde, 12.5g (0.201mol) ethylene glycol and 0.11g (0.58mmol) p-toluenesulfonic acid to 150ml toluene under the protection of argon. Reflux reaction for 25 hours, when no more water is produced in the water separator, stop heating, concentrate, wash with sodium bicarbonate and saturated brine, extract with dichloromethane, dry over anhydrous magnesium sulfate, spin dry, and distill under reduced pressure to obtain 2-(2-bromophenyl)-1,3-dioxolane 27.86g, yield 93%;
[0028] 2) Under argon protection, 27.8g (0.121mol) 2-(2-bromophenyl)-1,3-dioxolane, 2.96g (0.122mol) magnesium chips and 0.04g (0.157mmol) Add iodine to 200ml tetrahydrofuran, and reflux at 50°C for 2 hours to obtain 30.7g Grignard reagent;
[0029] 3) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com