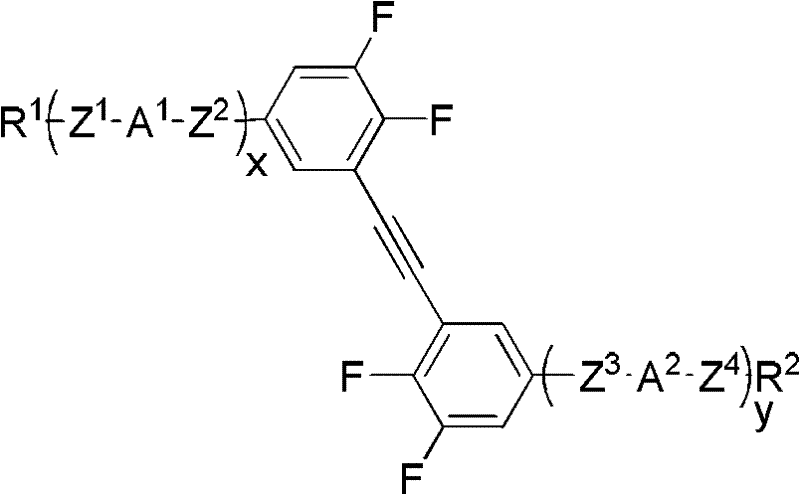
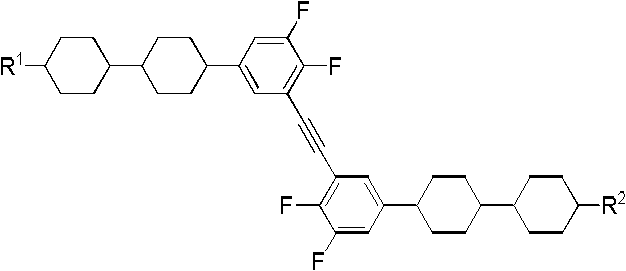
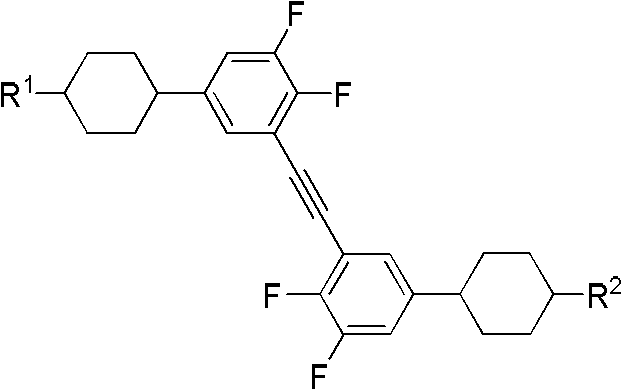
Side difluorine substituted diphenylacetylene liquid crystal compound, preparation method for same and application thereof
A compound, phenyl technology, applied in the field of lateral difluoro-substituted tolanyl acetylene liquid crystal compounds, can solve the problem of low resistivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1 compound formula I-1 (R 1 =C 2 h 5 , R 2 =C 2 h 5 ) preparation
[0083]
[0084] (Formula I-1)
[0085] Step 1: Preparation of 5-(4-(4'-ethylcyclohexyl)cyclohexyl)-2,3-difluoroiodobenzene
[0086]
[0087] In the 1000ml reaction bottle, drop into 0.10mol 5-(4-(4'-ethylcyclohexyl)cyclohexyl)-1,2-difluorobenzene, drop into 300ml THF, under N 2 Under protection, use a liquid nitrogen / ethanol bath to lower the temperature of the system below -80°C, add 0.15 moln-BuLi hexane solution dropwise, and keep it below -80°C to react for 1 hour after dropping. Maintain the temperature, and add dropwise a THF solution containing 0.15 mol of iodine. After the dropwise addition, keep the temperature for reaction for 1 hour, and then gradually rise to room temperature for reaction for 2 hours. Then add 100ml saturated aqueous sodium thiosulfate solution, separate the organic phase, wash 3 times with 30ml saturated brine, dry the organic phase with anhydrous s...
Embodiment 2
[0102] Embodiment 2 compound formula I-3 (R 1 =C 3 h 7 , R 2 =C 5 h 11 ) preparation
[0103]
[0104] (Formula I-3)
[0105] Step 1: Preparation of 5-(4-(4'-propylcyclohexyl)cyclohexyl)-2,3-difluoroiodobenzene
[0106] In the 1000ml reaction bottle, drop into 0.10mol 5-(4-(4'-propylcyclohexyl)cyclohexyl)-1,2-difluorobenzene, drop into 300ml THF, under N 2 Under protection, use a liquid nitrogen / ethanol bath to lower the temperature of the system below -80°C, add 0.15 moln-BuLi hexane solution dropwise, and keep it below -80°C to react for 1 hour after dropping. Maintain the temperature, and add dropwise a THF solution containing 0.15 mol of iodine. After the dropwise addition, keep the temperature for reaction for 1 hour, and then gradually rise to room temperature for reaction for 2 hours. Then add 100ml saturated aqueous sodium thiosulfate solution, separate the organic phase, wash 3 times with 30ml saturated brine, dry the organic phase with anhydrous sodium sul...
Embodiment 3
[0121] Embodiment 3 compound formula I-14 (R 1 =C 3 h 7 , R 2 =C 3 h 7 ) preparation
[0122]
[0123] (Formula I-14)
[0124] Step 1: Preparation of 2,3,3',4'-tetrafluoro-5'-iodo-4-propylbiphenyl
[0125] In the 1000ml reaction flask, drop into 0.10mol 2,3,3',4'-tetrafluoro-4-propyl biphenyl and 300ml THF, in N 2 Under protection, use a liquid nitrogen / ethanol bath to lower the temperature of the system below -80°C, add 0.15mol n-BuLi in hexane dropwise, and keep the temperature below -80°C to react for 1 hour after dropping. Maintain the temperature, and add dropwise a THF solution containing 0.15 mol of iodine. After the dropwise addition, keep the temperature for reaction for 1 hour, and then gradually rise to room temperature for reaction for 2 hours. Then add 100ml of saturated aqueous sodium thiosulfate solution, separate the organic phase, wash 3 times with 50ml of saturated brine, dry the organic phase with anhydrous sodium sulfate, and spin dry under reduc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Clear point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com