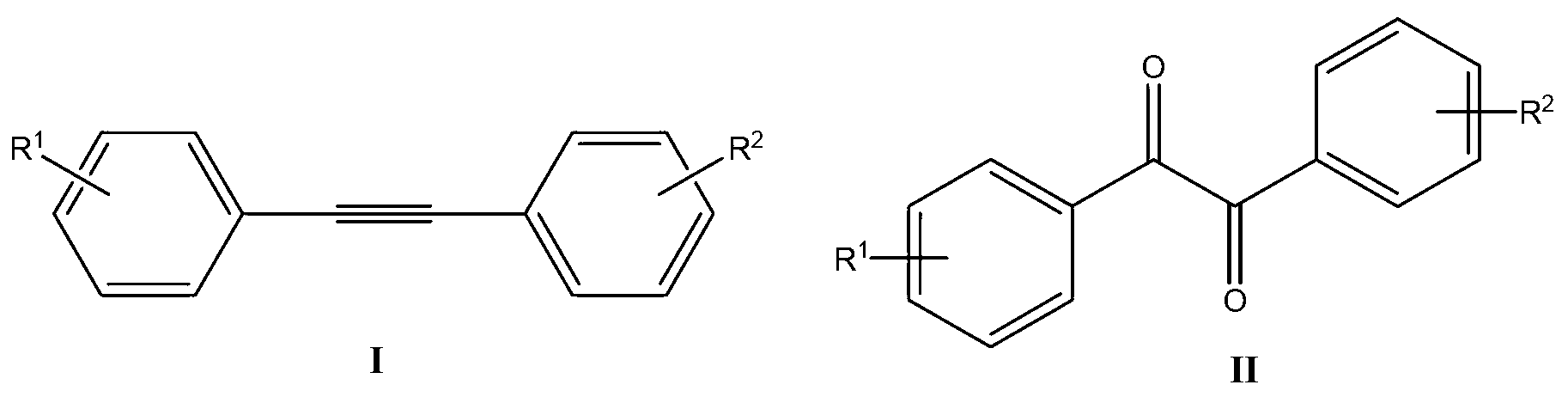
Synthetic method of benzil derivatives
A synthesis method and technology of derivatives, applied in chemical instruments and methods, preparation of organic compounds, preparation of carbonyl compounds by oxidation, etc., can solve the problem of expensive oxygen source, achieve cheap and easy-to-obtain environment, high yield, and substrate Universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
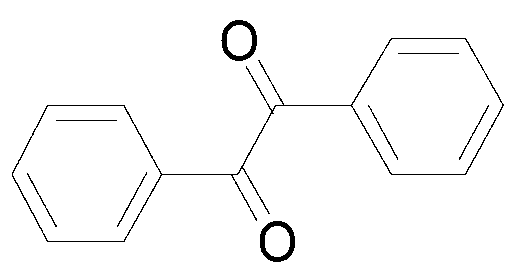
[0024] Add 35.6mg (0.2mmol) toluene, 141.7mg (0.4mmol) Selectfluor, 0.64mg (0.01mmol) Cu powder into a 10mL round bottom flask, then add 2mL acetonitrile / water (V:V=50:1) as a solvent. Then, the mixture was magnetically stirred for 4 h at room temperature. Then, 10 g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. :1) As an eluent, collect the eluate containing the product, and distill the eluate to remove the solvent to obtain the pure product. The material was a pale yellow oily liquid in 90% yield.
[0025] Characterization data: 1 H NMR (CDCl 3 ,500MHz):δ7.99(dd,J 1 =7.4Hz,J 2 =1.4Hz,4H),7.68(dd,J 1 =7.5Hz,J 2 =1.0Hz,2H),7.53(t,J=7.9,4H); 13 C N MR (CDCl 3 ,125MHz):δ194.6,134.9,133.1,129.9,129.0; m / z(%)=210(20)[M + ],105(100),77(56),51(40).
Embodiment 2
[0027]
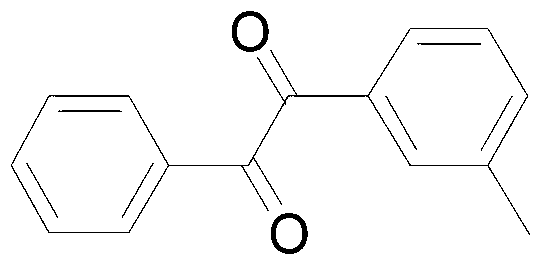
[0028] Add 38.4mg (0.2mmol3-methyltoluene), 141.7mg (0.4mmol) Selectfluor, 0.38mg (0.006mmol) Cu powder into a 10mL round bottom flask, then add 2mL acetonitrile / water (V:V=50 : 1) as solvent. Then, the mixture was magnetically stirred for 4 h at room temperature. Then, 10 g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. :1) As an eluent, collect the eluate containing the product, and distill the eluate to remove the solvent to obtain the pure product. The material was a pale yellow oily liquid in 78% yield.
[0029] Characterization data: 1 H NMR (CDCl 3,500MHz):δ=7.98(d,J=7.5Hz,2H),7.89(d,J=8.0Hz,2H),7.67(t,J=7.5Hz,1H),7.52(t,J=7.5Hz ,2H),7.32(d,J=8.0Hz,2H),2.45(s,3H);MS(EI,70eV):m / z(%)=224(3)[M + ],119(100).
Embodiment 3
[0031]
[0032] Add 38.4mg (0.2mmol) of 2-methyltoluene, 141.7mg (0.4mmol) of Selectfluor, 0.64mg (0.01mmol) of Cu powder into a 10mL round bottom flask, and then add 2mL of acetonitrile / water (V:V= 50:1) as solvent. Then, magnetic stirring was carried out at room temperature for 8 h. Then, 10 g of column chromatography silica gel (100-200 mesh) was added to the reaction solution, and the solvent was removed by distillation under reduced pressure. :1) As an eluent, collect the eluate containing the product, and distill the eluate to remove the solvent to obtain the pure product. The material was a pale yellow oily liquid, 64% yield.
[0033] Characterization data: 1 H NMR (CDCl 3 ,500MHz):δ=8.0(d,J=7.0Hz,2H),7.68(t,J=7.5Hz,2H),7.55-7.29(m,5H),2.73(s,3H); MS(EI, 70eV):m / z(%)=224(3)[M + ],119(100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com