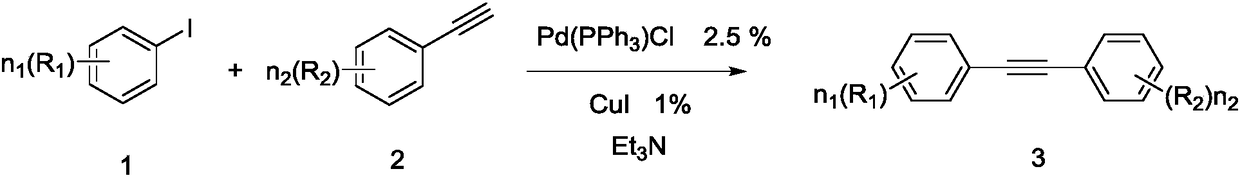
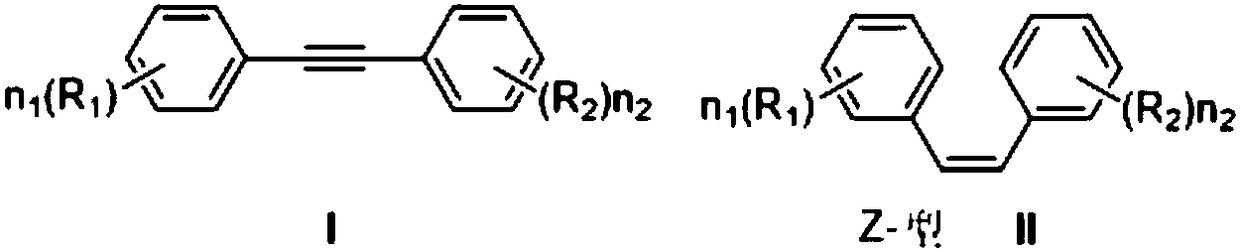Method for highly selectively reducing alkyne to generate Z-olefin
A high-selectivity, synthetic method technology, applied in the field of organic compound synthesis, can solve problems such as few researches, and achieve the effects of high selectivity, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] 0.02mmol cuprous chloride (2mg), 0.03mmol 1,3-bis(2,4,6-trimethylphenyl) imidazolium chloride (10.3mg), 0.2mmol potassium tert-butoxide (22.4mg) Put it into a Schlenk reaction tube, vacuumize it, and replace the nitrogen three times, add 1ml of ethanol as a solvent, and stir at room temperature for 15 minutes. Then 0.2 mmol of diphenylacetylene (35.6 mg) and 0.24 mmol of diboronic acid pinacol ester (61 mg) were dissolved in 2 mL of ethanol, and then added dropwise into the reaction tube, and stirred at room temperature for 5 hours. After the reaction was finished, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure. The mixture of esters is used as the eluent for elution, and the elution process is tracked by TLC, the eluent containing the target product is collected, and the eluent is combined to evaporate the solvent to obtain the pure product. The material was...
Embodiment 2
[0033]
[0034] 0.01 mmol cuprous chloride (1 mg), 0.02 mmol 1,3-bis(2,4,6-trimethylphenyl) imidazolium chloride (6.9 mg), 0.2 mmol potassium tert-butoxide (22.4 mg) Put it into a Schlenk reaction tube, vacuumize it, and replace the nitrogen three times, add 1ml of ethanol as a solvent, and stir at room temperature for 15 minutes. Then 0.2 mmol of diphenylacetylene (35.6 mg) and 0.24 mmol of pinacol diborate (61 mg) were dissolved in 2 mL of ethanol, and then added dropwise into the reaction tube, and stirred at room temperature for 5 hours. After the reaction was finished, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure. The mixture of esters is used as the eluent for elution, and the elution process is tracked by TLC, the eluent containing the target product is collected, and the eluent is combined to evaporate the solvent to obtain the pure product. The material was a colo...
Embodiment 3
[0037]
[0038] Add 0.005mmol cuprous chloride (0.5mg), 0.01mmol 1,3-bis(2,4,6-trimethylphenyl) imidazolium chloride (3.4mg), 0.2mmol potassium tert-butoxide (22.4mg ) into the Schlenk reaction tube, evacuated, and filled with nitrogen for 3 times, added 1ml of ethanol as a solvent, and stirred at room temperature for 15 min. Then 0.2mmol of diphenylacetylene (35.6mg) and 0.24mmol of pinacol diborate (61mg) were dissolved in 2mL of ethanol, and added dropwise to the reaction tube, and stirred at room temperature for 5 hours. After the reaction was finished, 100-200 mesh column chromatography silica gel was added to the obtained reaction solution and the solvent was distilled off under reduced pressure. The mixture of esters is used as the eluent for elution, TLC tracks the elution process, collects the eluent containing the target product, combines the eluents and evaporates the solvent to obtain a pure product. The material was a colorless liquid, 55% yield.
[0039] Cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com