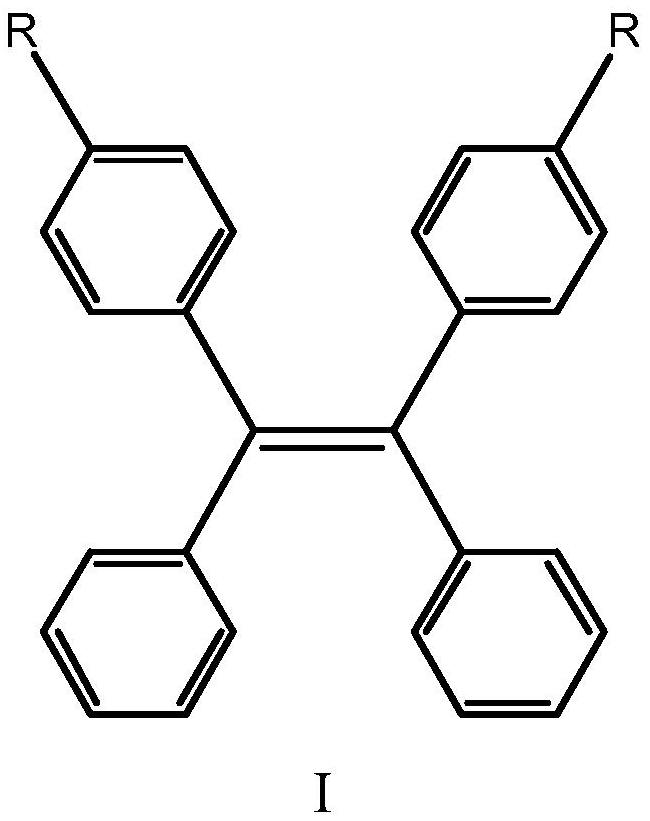
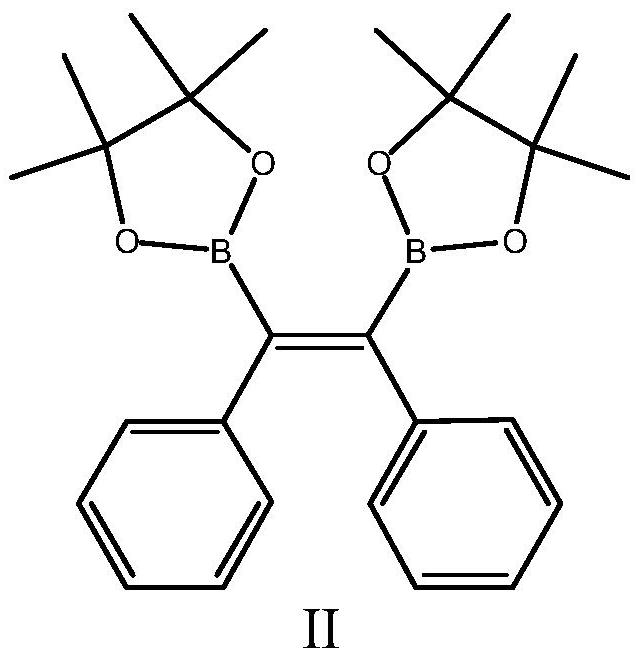
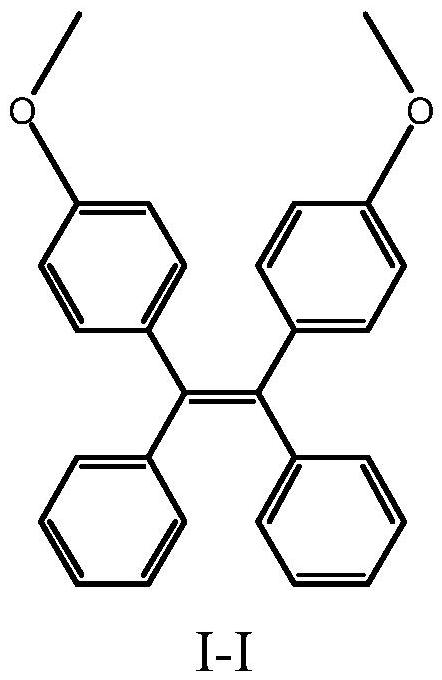
Piezochromic tetraphenylethylene compound as well as preparation method and application thereof
A tetraphenylethylene and piezochromic technology, applied in the field of fluorescent materials, can solve the problems of unusual emission phenomenon of TPE-based polymers, complex synthesis process, harsh synthesis conditions, etc., achieve strong molecular aggregation-induced luminescent effect, and improve reaction efficiency , color sensitive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a method for preparing tetrastyryl cis-position diether, which specifically includes the following steps:
[0030] Step a, add 10.2g (40mmol) pinacol diborate and 0.25g (0.2mmol) tetrakistriphenylphosphine platinum into a dry reaction vessel containing 50ml N,N-dimethylformamide and nitrogen gas , then dissolve 3.6g (20mmol) toluene in 50ml N,N-dimethylformamide to form a solution, drop it into the above reaction vessel, raise the temperature to 90°C, react at 90°C for 9h, and cool to Room temperature, rotary evaporation under reduced pressure to obtain the crude product solution of distyryl borate pinacol ester, after column chromatography (stationary phase is silica gel) with petroleum ether: dichloromethane = 2:1 as the eluent, the washing The solvent was removed by rotary evaporation under reduced pressure to obtain 5.95 g of white needle-like solid, that is, refined distyrylboronic acid pinacol ester, with a yield of 85% and a purity of 98%...
Embodiment 2
[0040] This embodiment provides a preparation method of tetrastyryl dimethyl, which specifically includes the following steps:
[0041] Step a, add 10.2g (40mmol) pinacol diborate and 0.25g (0.2mmol) tetrakistriphenylphosphine platinum into a dry reaction vessel containing 50ml N,N-dimethylformamide and nitrogen gas , then dissolve 3.6g (20mmol) toluene in 50ml N,N-dimethylformamide to make a solution, drop it into the above reaction vessel, raise the temperature to 85°C, react at 85°C for 9.5h, cool to room temperature, rotary evaporation under reduced pressure to obtain the crude product solution of distyryl borate pinacol ester, after column chromatography (stationary phase is silica gel) with petroleum ether: dichloromethane = 2:1 as the eluent, the The solvent was removed from the eluate by rotary evaporation under reduced pressure to obtain 5.6 g of white needle-like solid with a yield of 80%.
[0042] Step b, take 3.50g (10mmol) refined distyrylboronic acid pinacol est...
Embodiment 3
[0051] This embodiment provides a kind of preparation method of tetrastyryl cis-position diester, specifically comprises the following steps:
[0052] Step a, add 10.2g (40mmol) pinacol diborate and 0.25g (0.2mmol) tetrakistriphenylphosphine platinum into a dry reaction vessel containing 50ml N,N-dimethylformamide and nitrogen gas , then dissolve 3.6g (20mmol) toluene in 50ml N,N-dimethylformamide to make a solution, drop it into the above reaction vessel, raise the temperature to 70°C, react at 70°C for 10h, and cool to Room temperature, rotary evaporation under reduced pressure to obtain the crude product of distyrylboronic acid pinacol ester, after column chromatography (stationary phase is silica gel) with petroleum ether: dichloromethane = 2:1 as the eluent, the eluted The solvent was removed by rotary evaporation under reduced pressure to obtain 4.9 g of white needle-like solid with a yield of 70%.
[0053] Step b, take 2.43g (6.94mmol) refined distyrylboronic acid pina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com