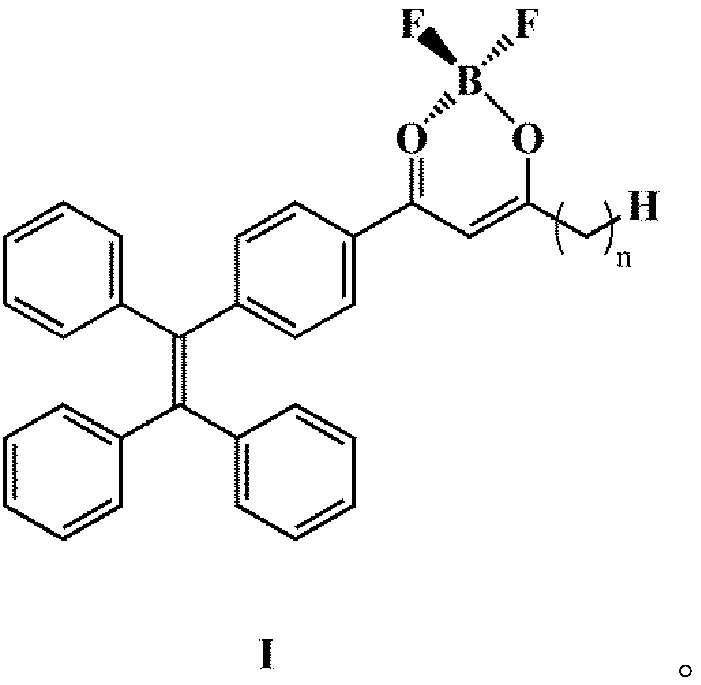
Alkyl side-chain type phenyl boron fluoride complexes as well as preparation method and application thereof
A chain phenyl boron complex technology, applied in the alkyl side chain phenyl boron fluoride complex and its preparation method and application field, can solve the problem of few piezochromic materials, harsh synthesis conditions, complex synthesis process, etc. problems, to achieve the effect of short reaction cycle, broaden the application market, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
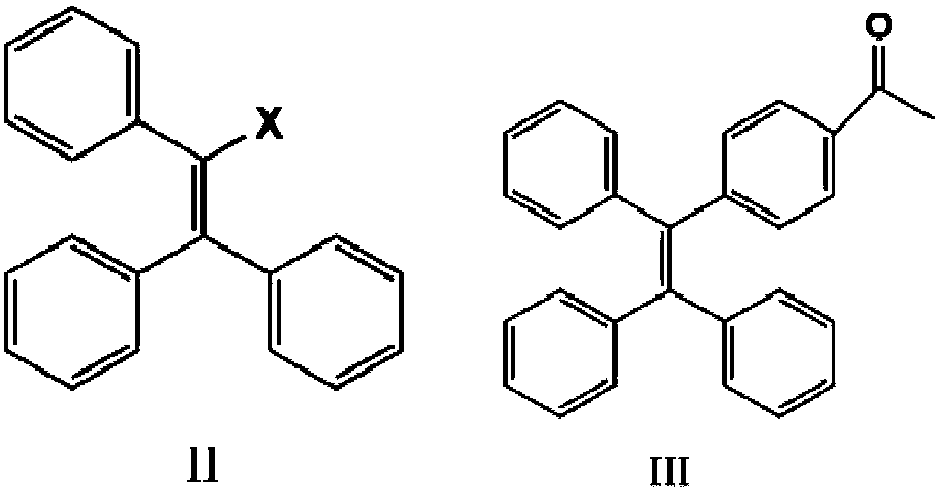
[0031] (1) Combine 7.5g (22.35 mmol) 2-bromo-1,1,2-tristyrene (II) with 3.75g (22.85 mmol) 4-acetylphenylboronic acid and 0.05g (0.0435 mmol) four Triphenylphosphine palladium, 5.5g (39.8 mmol) potassium carbonate, 3.61g (11.2 mmol) tetrabutylammonium bromide were added to a dry reaction vessel bubbling with nitrogen, 80ml of toluene was added as a solvent, and heated to reflux at 100°C for 24 After hours, cool to room temperature, extract the crude product of tetrastyryl monoketone (Ⅲ) with dichloromethane, and perform column chromatography with petroleum ether: dichloromethane=1:1 to obtain 6.68g white solid with a yield of 80% .
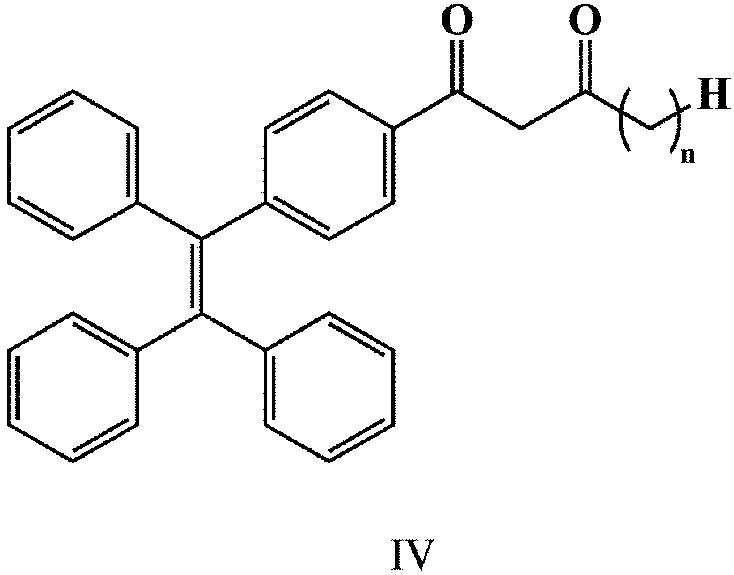
[0032] (2) Add 1.56g (4mmol) of the white solid and 0.37g (4mmol) of methyl propionate into a dry reaction vessel vented with nitrogen, add 30ml of tetrahydrofuran as a solvent, and add the white solid after the white solid is completely dissolved. 0.3975g (16.5 mmol) of sodium hydride, heated to reflux at 50°C, reacted for 4 hours, cooled to room t...
Embodiment 2
[0035] (1) Combine 7.5g (22.39 mmol) 2-bromo-1,1,2-tristyrene (II) with 4.03g (24.58 mmol) 4-acetylphenylboronic acid and 0.06g tetrakistriphenylphosphine palladium , 5.96g potassium carbonate, 3.97g tetrabutylammonium bromide were added to a dry reaction vessel filled with nitrogen, 80ml xylene was added, heated to reflux at 100°C for 24 hours, cooled to room temperature, and tetrastyrene was extracted with dichloromethane The crude product of base monoketone (III), petroleum ether: dichloromethane=1:1, was subjected to column chromatography to obtain 6.71 g of white solid with a yield of 80%.
[0036] (2) Add 1.56g (4mmol) of the white solid and 1.11g (12mmol) of methyl propionate into a dry reaction vessel with nitrogen, add 30ml of solvent dichloromethane, and wait until the white solid is completely dissolved , Add 0.3975g (16.5 mmol) sodium hydride, heat to reflux at 55°C, react for 3 hours, cool to room temperature, spin-evaporate the product to slightly dry, petroleum eth...
Embodiment 3
[0039] (1) Combine 7.5g (22.39 mmol) 2-bromo-1,1,2-tristyrene (II) with 3.75g (22.87 mmol) 4-acetylphenylboronic acid and 0.05g (0.0435 mmol) four Triphenylphosphine palladium, 5.5g (39.8 mmol) potassium carbonate, 3.61g (11.2 mmol) tetrabutylammonium bromide are added to a dry reaction vessel with nitrogen, 80ml of toluene is added, and it is heated to reflux at 100°C for 24 hours After cooling to room temperature, the crude product of tetrastyryl monoketone (III) was extracted with dichloromethane, and column chromatography was performed on petroleum ether: dichloromethane=1:1 to obtain 6.68 g of white solid with a yield of 80%.
[0040] (2) Add 1.56g (4mmol) of the white solid and 0.413g (4mmol) of methyl butyrate into a dry reaction vessel vented with nitrogen, add 30ml of solvent tetrahydrofuran, and after the white solid is completely dissolved, add 0.3975g (16.5 mmol) of sodium hydride, heated to reflux at 60°C, reacted for 5 hours, cooled to room temperature, the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com