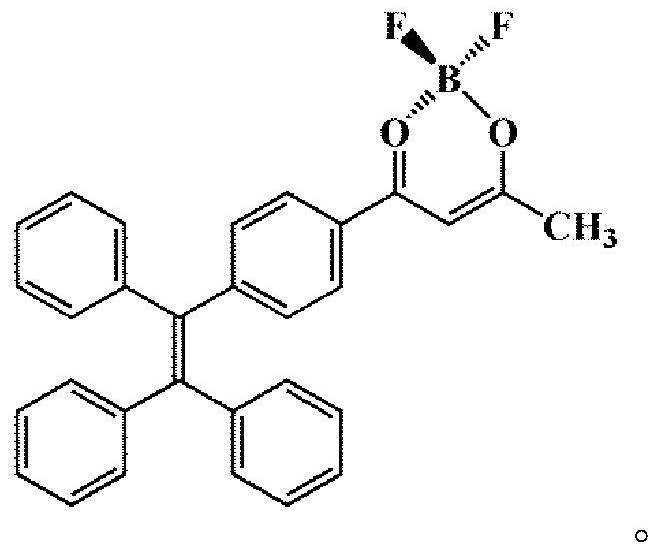
A kind of phenylboron fluorine complex and its preparation method and application
A technology of phenyl boron and complexes, which is applied in the field of phenyl boron and fluorine complexes and its preparation, can solve the problems of dangerous environmental pollution, less frictional discoloration materials, complex synthesis process, etc., shorten the reaction time, The effect of short reaction cycle and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of 1-(4-(1,2,2-triphenylethenyl) phenyl) ethanone (tetrastyryl monoketone, III)
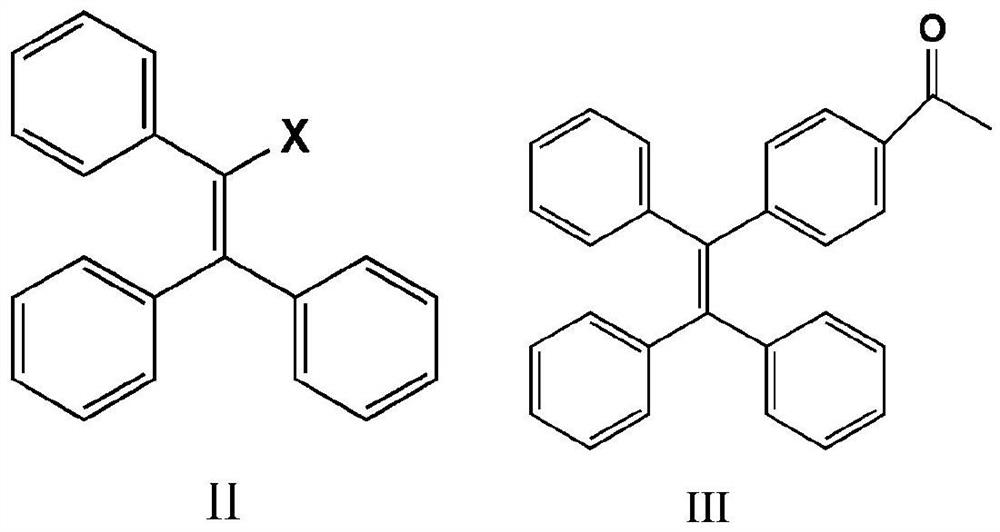
[0030] At room temperature, mix 15g (44.7 mmol) 2-bromo-1,1,2-triphenylethylene (II) with 7.5 g (45.7 mmol) 4-acetylphenylboronic acid and 0.1 g (0.087 mmol) tetratriphenyl Phosphine palladium, 11g (79.6 mmol) of potassium carbonate, 7.21g (22.4 mmol) of tetrabutylammonium bromide were added to a dry reaction vessel with nitrogen, 160ml of toluene was added, heated to reflux at 100°C for 24 hours, and cooled to At room temperature, dichloromethane was used to extract the crude product of tetrastyryl monoketone (III). Column chromatography was performed on petroleum ether:dichloromethane=1:1 to obtain 13.40 g of white solid, with a yield of 80%.
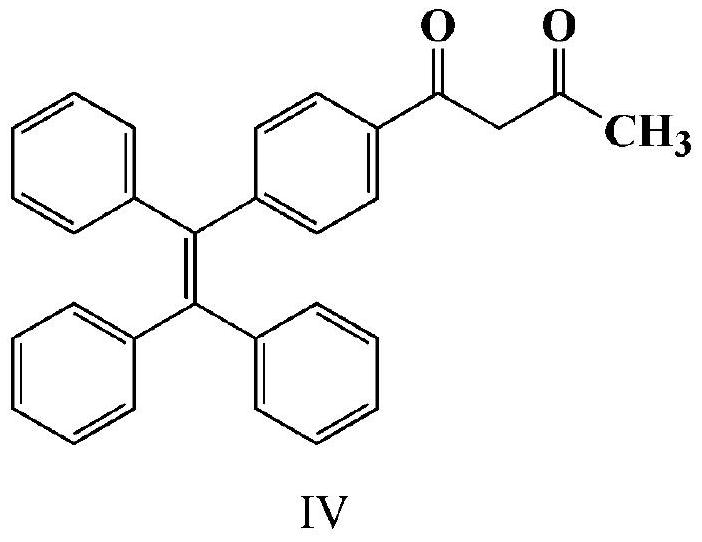
[0031] (2) Synthesis of 3-hydroxyl-1-(4-(1,2,-triphenylvinyl)phenyl)but-2-en-1-one (tetrastyryl β-diketone, Ⅳ)
[0032] Add 1.56g (4 mmol) of the above white solid and 100ml of ethyl acetate into a dry reaction vessel with nitrogen ...
Embodiment 2
[0036] Except step (1) and (2), other is with embodiment 1, and the synthetic technique of step (1), (2) is as follows:
[0037] (1) Mix 7.5g (22.39mmol) of 2-bromo-1,1,2-triphenylethylene (II) with 4.03g (24.58mmol) of 4-acetylphenylboronic acid and 0.06g of tetrakistriphenylphosphine palladium , 5.96g of potassium carbonate and 3.97g of tetrabutylammonium bromide were added to a dry reaction vessel with nitrogen, 80ml of xylene was added, heated to reflux at 100°C for 24 hours, cooled to room temperature, and tetraphenylethylene was extracted with dichloromethane The crude product of monoketone (Ⅲ), petroleum ether: dichloromethane = 1:1, was subjected to column chromatography to obtain 6.71 g of white solid with a yield of 80%.
[0038](2) Add 1.56g (4 mmol) of the above-mentioned white solid and 100ml of ethyl acetate into a dry reaction vessel with nitrogen gas, and after the white solid is completely dissolved, add 0.3975g (16.5 mmol) of sodium hydride at 40°C Heated, r...
Embodiment 3
[0040] Except step (2), other is with embodiment 1, and the synthesis technique of step (2) is as follows:
[0041] Add the above-mentioned 1.56g (4 mmol) white solid and 100ml ethyl acetate into a dry reaction vessel with nitrogen, and after the white solid is completely dissolved, add 0.3975g (16.5 mmol) sodium hydride, heat at 50°C, and react After 6 hours, it was cooled to room temperature, and the ethyl acetate in the product was evaporated to dryness, petroleum ether: dichloromethane = 2:1, and purified by column chromatography, and the obtained solid was directly used in the next step of synthesis reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com