Aromatic diamine containing phthalonitrile side group and synthesis method and application thereof
A technology of phthalonitrile and aromatic diamine, applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc. The effect of improved properties such as thermal properties and solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
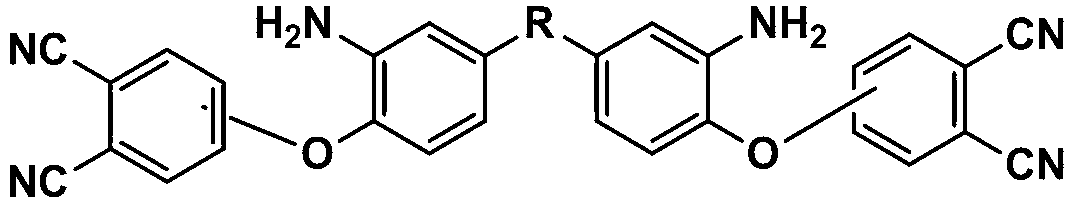
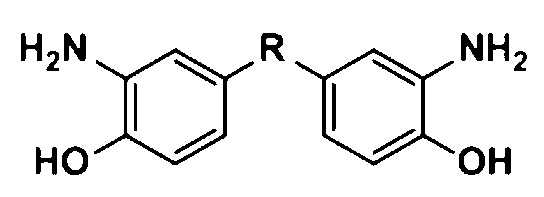
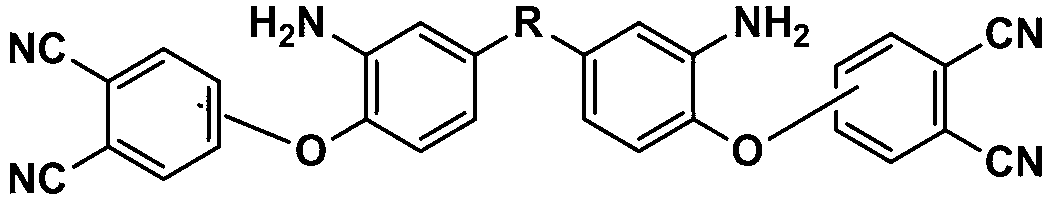
[0044] Example 1: Synthesis of aromatic diamine 2a containing phthalonitrile side groups (R in the structural formula is CF3CCF3)
[0045] Raw materials include 4-nitrophthalonitrile, 1a, potassium carbonate, and dimethylsulfoxide, wherein the molar ratio of 4-nitrophthalonitrile to 1a is 2:1, and the molar ratio of potassium carbonate to 1a The ratio is 3:1, the volume of dimethyl sulfoxide: the sum of the mass of 4-nitrophthalonitrile and 1a is 10:2, the unit of measurement of the volume is mL, and the unit of measurement of the mass is g.
[0046] Add 4.73g (27.3mmol) 4-nitrophthalonitrile, 5g (13.65mmol) 1a, 5.67g (40.95mmol) potassium carbonate, 48.7ml dimethyl sulfoxide to the reaction vessel, under nitrogen protection, React at 5°C for 24h. After the reaction, pour the reaction solution into tap water to precipitate, then filter, wash the obtained filter cake with tap water until neutral, and dry it under vacuum at 50°C for 24h to obtain 2a with a yield of 90.3%.
[0...
Embodiment 2
[0048] Example 2: Synthesis of aromatic diamine 2b containing phthalonitrile side groups (R in the structural formula is CH3CCH3)
[0049] Raw materials include 3-nitrophthalonitrile, 1b, potassium carbonate, N,N-dimethylformamide, wherein the molar ratio of 3-nitrophthalonitrile to 1b is 2.05:1, potassium carbonate The molar ratio to 1b is 3.5:1, the volume of N,N-dimethylformamide: the sum of the masses of 3-nitrophthalonitrile and 1b is 10:2.2, and the unit of measurement for said volume is mL , the unit of measurement of the mass is g.
[0050] Add 5.5 g (31.73 mmol) 3-nitrophthalonitrile, 4 g (15.48 mmol) 1b, 7.49 g (54.18 mmol) potassium carbonate, 43.2 ml N,N-dimethylformamide to the reaction vessel under nitrogen atmosphere Under protection, react at 15°C for 20h. After the reaction, pour the reaction solution into deionized water to precipitate, then filter, wash the obtained filter cake with deionized water until neutral, and vacuum dry at 60°C for 20h to obtain 2b....
Embodiment 3
[0052] Example 3: Synthesis of aromatic diamine 2c containing phthalonitrile side groups (R in the structural formula is O)
[0053] Raw materials include 4-nitrophthalonitrile, 1c, potassium carbonate, N,N-dimethylacetamide, wherein the molar ratio of 4-nitrophthalonitrile to 1c is 2.1:1, potassium carbonate The molar ratio to 1c is 4:1, the volume of N,N-dimethylacetamide: the sum of the masses of 4-nitrophthalonitrile and 1c is 10:2.5, and the unit of measurement for said volume is mL , the unit of measurement of the mass is g.
[0054] Add 15.66 g (90.43 mmol) 4-nitrophthalonitrile, 10 g (43.06 mmol) 1c, 23.8 (172.24 mmol) g potassium carbonate, 102.6 ml N,N-dimethylacetamide to the reaction vessel under nitrogen atmosphere Under protection, react at 20°C for 15h. After the reaction, pour the reaction solution into deionized water to precipitate, then filter, wash the obtained filter cake with deionized water until neutral, and dry it in vacuum at 80°C for 10h to obtain 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com