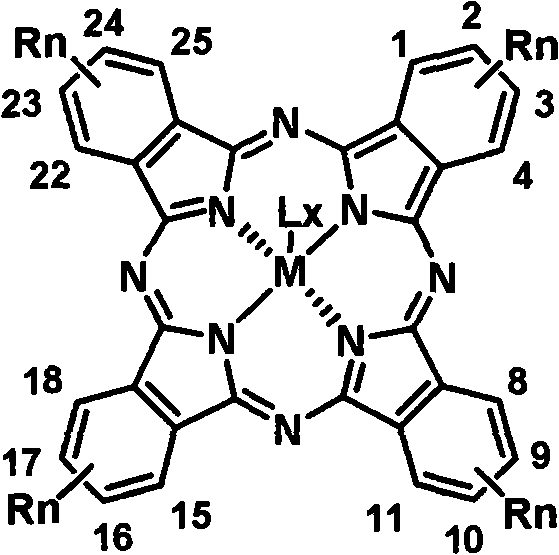
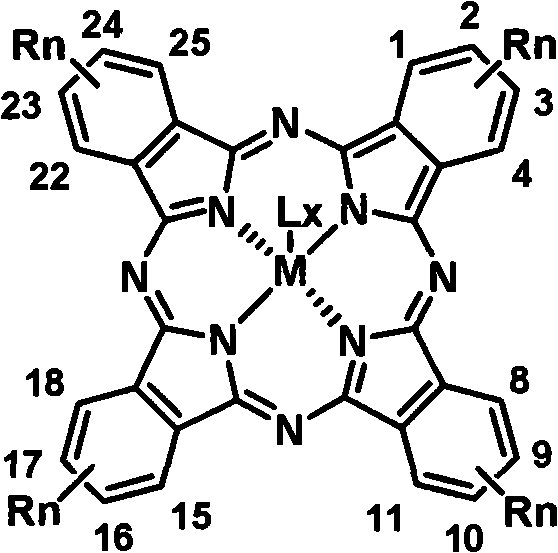
Soluble tetraalkyl phthalocyanine compound and preparation method thereof
A tetraalkyl phthalocyanine and soluble technology, which is applied in the field of soluble tetraalkyl phthalocyanine compounds and their preparation, can solve the problems of high cost, harsh conditions, complicated processing methods, etc., and achieves simple structure, mild reaction conditions and good dissolution. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: 4-(1-Butynyl)phthalonitrile
[0029] Add 4-iodophthalonitrile (2.1g, 8.0mmol), CuI (0.61g, 3.2mmol) and Pd(PPh) to a 150ml ampoule 3 ) 4 (0.23g, 0.32mmol) The gas was replaced with high-purity nitrogen three times, and a mixed solvent of 80 ml of triethylamine and 20 ml of tetrahydrofuran was added to it. Freeze 1-butyne into a liquid in an ice-salt bath, use a syringe to extract 1-butyne (0.86g, 16mmol) into the above ampoule, seal the tube, stir at room temperature for 24 hours, stop the reaction, pour 400ml In ether, extract with enough ammonium chloride solution until the pH of the organic layer is 7, wash with saturated brine, dry with anhydrous magnesium sulfate, and rinse with a mixture of petroleum ether / ethyl acetate with a volume ratio of 10:1 The reagent was separated by column chromatography to obtain 1.2 g of 4-(1-butynyl)phthalonitrile with a yield of 85%. NMR characterization data: 1 H NMR(300MHz, CDCl 3 ) δ (ppm) 7.74 (d, 1H), 7.71 (d, 1H), 7.68 (...
Embodiment 2
[0030] Example 2: 4-(1-octynyl)phthalonitrile
[0031] 4-iodophthalonitrile (2.1g, 8.0mmol), CuI (0.61g, 3.2mmol) and Pd(PPh 3 ) 4 (0.23g, 0.32mmol) was added to a 150ml reaction flask, the gas was replaced with high-purity nitrogen three times, and a dried mixed solvent of 80ml triethylamine and 20ml tetrahydrofuran was added to the reaction flask. Finally, add n-octyne (1.5g, 10mmol), react at room temperature for 17 hours, stop the reaction, pour it into 500 ml of ether, extract with enough ammonium chloride solution until the organic layer pH is 7, and wash with saturated brine. Drying with water magnesium sulfate, using a mixture solvent of petroleum ether / ethyl acetate with a volume ratio of 10:1 as the eluent for column chromatography to obtain 1.8 g of 4-(1-octynyl)phthalonitrile. The rate is 97%. NMR characterization data: 1 H NMR(300MHz, CDCl 3 )δ(ppm) 7.75(d, 1H), 7.70(d, 1H), 7.66(dd, 1H), 2.44(t, 2H), 1.60(m, 2H), 1.42(m, 2H), 1.31(m , 4H), 0.90 (t, 3H).
Embodiment 3
[0032] Example 3: 4-(1-Dodecynyl)phthalonitrile
[0033] Using n-dodecyne hydrocarbon instead of n-octyne in Example 2, the feed ratio, reaction conditions and treatment methods were the same as those in Example 2, to obtain 4-(1-dodecynyl)phthalonitrile with a yield of 95%. NMR characterization data: 1 H NMR(300MHz, CDCl 3 )δ(ppm) 7.75(d, 1H), 7.69(d, 1H), 7.64(dd, 1H), 2.40(t, 2H), 1.62(m, 2H), 1.42(m, 2H), 1.30(m , 8H), 0.90 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com