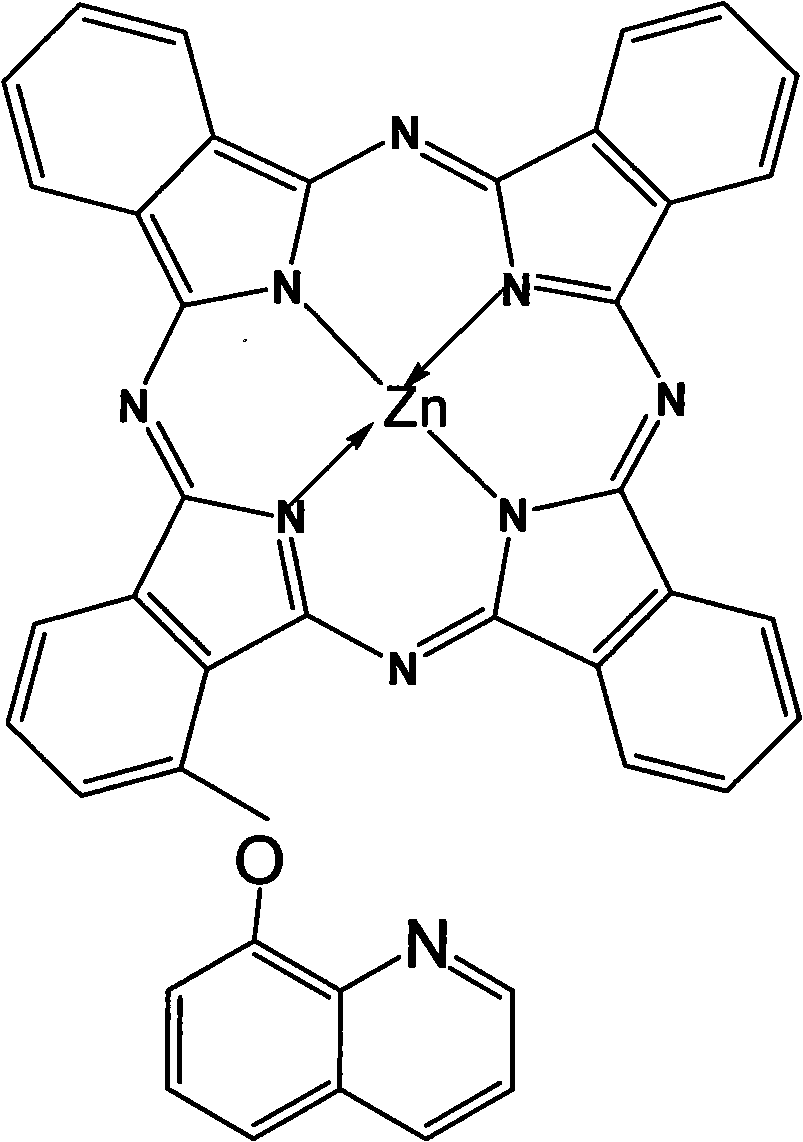
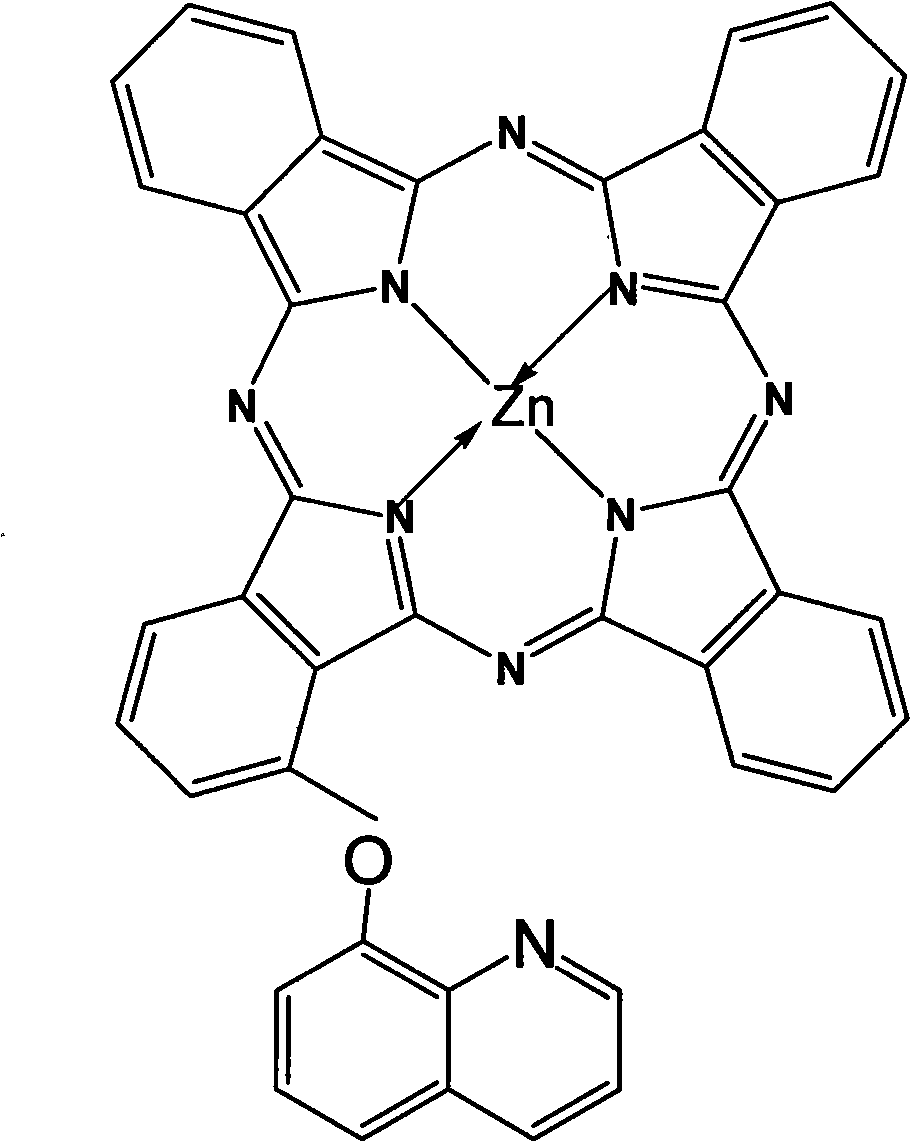
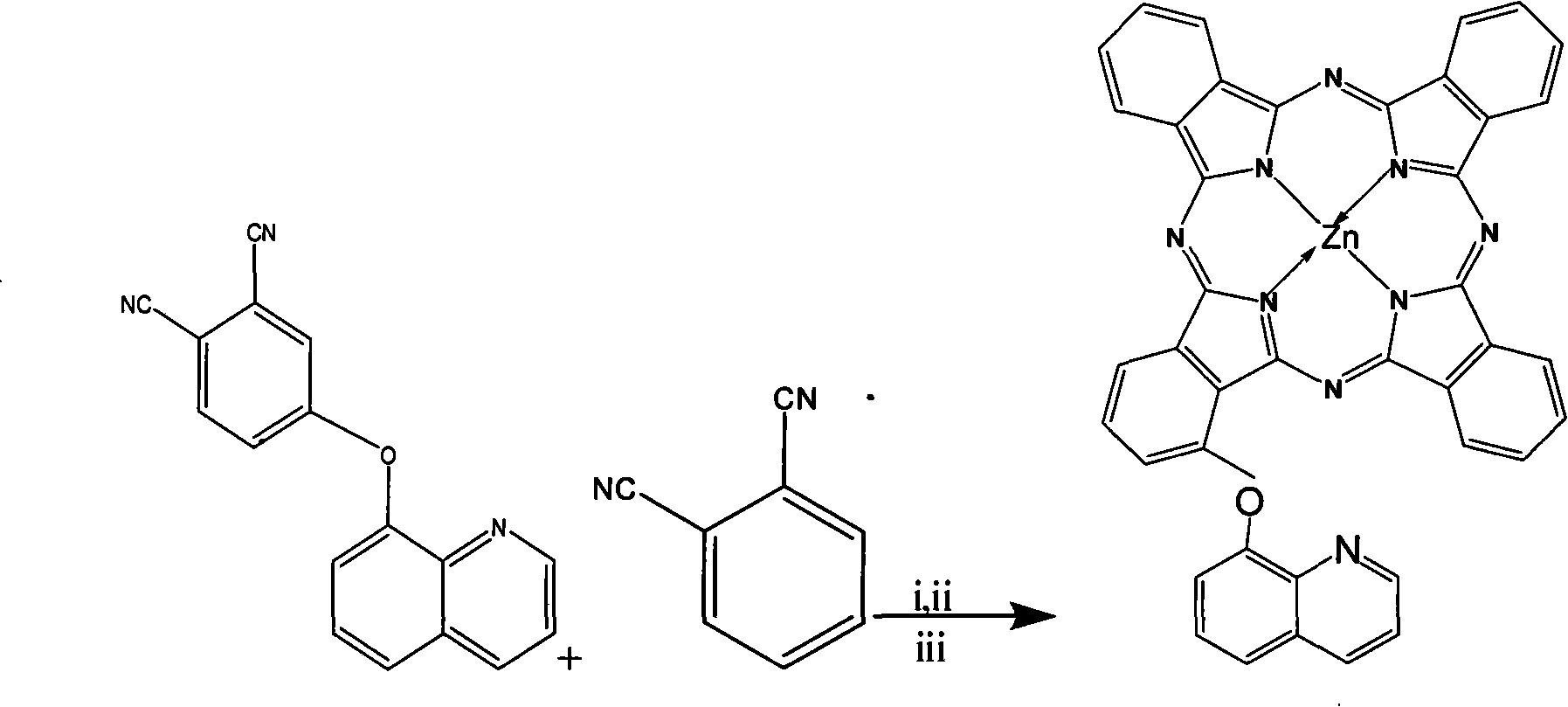
Alpha-(8-quinolineoxy)mono-substituted zinc phthalocyanine and preparation method thereof
A technology of quinolineoxyl and zinc phthalocyanine, applied in zinc organic compounds, organic chemistry, etc., can solve the problems of many side reactions, difficult separation, difficult synthesis, etc., achieve less side reactions, easy to obtain raw materials, and comprehensive synthesis method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The steps of the preparation method are:
[0013] (1) Synthesis of 3-(8-quinolineoxy) phthalonitrile: in the three-necked bottle equipped with a magnetic stirring device, an airway, and a reflux device, add successively dry 3-nitro-o-phthalonitrile according to the molar ratio Phthalonitrile and 8-hydroxyquinoline 1~8: 1~6, and add potassium carbonate and 10~30ml: the amount of 1mol8-hydroxyquinoline adds solvent N according to the amount of 1~6mol: 1mol8-hydroxyquinoline, N-Dimethylformamide DMF, stirred, and reacted at 5-40°C for 3-12 hours under the protection of nitrogen; after the reaction, pour it into ice water, stir it thoroughly and filter it with suction, and wash the filter cake with distilled water to the filtrate The obtained solid is recrystallized several times with methanol to obtain a pink solid; the pink solid is dried and weighed, the yield is 75-85%, and the melting point is 212-213°C;
[0014] (2) Synthesis of zinc phthalocyanine complex: In a reac...
Embodiment 1
[0019] (1) Synthesis of 3-(8-quinolineoxy)phthalonitrile: Add dry 1.38g (8mmol) 3-nitrate in turn in a 100ml three-necked bottle equipped with a magnetic stirring device, an airway, and a reflux device Base phthalonitrile and 1.16g (8mmol) 8-hydroxyquinoline, 1g potassium carbonate and 10mlDMF, stirred, and reacted at 10°C for 5 hours under the protection of nitrogen. After the reaction was finished, it was poured into an appropriate amount of ice water, stirred thoroughly, and suction filtered, and the filter cake was washed with distilled water until the filtrate was neutral. The obtained solid was recrystallized several times with methanol to obtain a pink solid. After drying and weighing, the yield was 81%, and the melting point was 212-213°C.
[0020] (2) Synthesis of zinc phthalocyanine complex: Add 0.28g (1mmol) 3-(8-quinolineoxy) phthalonitrile and 1.15g (9mmol) o-phthalonitrile in sequence in a reaction vessel with a reflux condensing device Diformonitrile and 0.3ml ...
Embodiment 2
[0025] The preparation method is the same as in Example 1, in (1) process, the mol ratio of 3-nitrophthalonitrile and 8-hydroxyquinoline is changed into 2: 1, other operations are identical, 3-(8-quinoline The productive rate of oxygen) phthalonitrile is 78%; In (2) process, the mol ratio of 3-(8-quinolineoxy) phthalonitrile and phthalonitrile is changed into 1: 5. Other operations are the same as in Example 1, and the yield of the final target product is 26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com