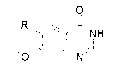
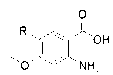
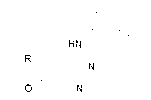
Erlotinib-phthalocyanine conjugate as molecule-targeting anticancer photosensitizer
A molecular targeting and photosensitizer technology, applied in the field of molecular targeting anticancer photosensitizer erlotinib-phthalocyanine conjugate and preparation thereof, can solve the problem that tumor tissue targeting is not ideal, separation is difficult, and Synthesis difficulties and other problems, to achieve the effects of low cost, easy availability of raw materials, and simple synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 (M=Zn, m=3, R=-CH 3 O, α-position monosubstituted)
[0045] 1) Add 1.0g (5.07mmol) of the compound and 1.0g (10mmol) of formamidine acetate into 20ml of ethanol solution, and microwave for 3h. After the reaction was completed, the ethanol was spin-dried, and then extracted with dichloromethane and water to obtain a crude product. After using dichloromethane-methanol as the eluent through a silica gel column, the compound is isolated 2。
[0046] ) , 1 g (4.8 mmol) of the compound 2 Add 1.48g (9.7mmol) phosphorus oxychloride, react for 4-9h, remove the remaining phosphorus oxychloride by rotary evaporation, and then extract with water and dichloromethane to obtain crude product. The compound was obtained by passing through a silica gel column 3 .
[0047] 3), 1g (4.46mmol) compound 3 and 1 g (8.92 mmol) of 3-aminophenylacetylene were added to isopropanol. After reacting for 4-9h, spin the isopropanol to dryness, then use dichloromethane-methanol as t...
Embodiment 2
[0052] Example 2 (M=Al, m=7, R=-H, α-position monosubstituted)
[0053] 1) Add 1g (mmol) of the compound and 1.0g (mmol) of formamidine acetate into 20ml of ethanol solution, and microwave for 3h. After the reaction was completed, the ethanol was spin-dried, and then extracted with dichloromethane and water to obtain a crude product. After using dichloromethane-methanol as the eluent through a silica gel column, the compound is isolated 2。
[0054] 2), 1g (mmol) compound 2 Add 1.48g (mmol) phosphorus oxychloride, react for 4-9h, remove the remaining phosphorus oxychloride by rotary evaporation, and then extract with water and dichloromethane to obtain crude product. After passing through a silica gel column to obtain the compound 3 .
[0055] 3), 1g (mmol) compound 3 and 1 g (mmol) of 3-aminophenylacetylene were added to isopropanol. After reacting for 4-9 hours, the isopropanol was spin-dried, and then dichloromethane-methanol was used as the eluent, and the compound w...
Embodiment 3
[0060] Embodiment 3 (M=Si, m=1, R=-CH 2 OCH 2 CH 2 OCH 3 , α-position monosubstituted)
[0061] 1), 1.0g compound 1 And 1.0g of formamidine acetate was added to 20ml of ethanol solution, microwave reaction for 3h. After the reaction was completed, the ethanol was spin-dried, and then extracted with dichloromethane and water to obtain a crude product. After using dichloromethane-methanol as the eluent through a silica gel column, the compound is isolated 2。
[0062] ) , the 1g compound 2 Add 1.51g of phosphorus oxychloride, react for 4-9h, remove the remaining phosphorus oxychloride by rotary evaporation, and then extract with water and dichloromethane to obtain the crude product. After passing through a silica gel column to obtain the compound 3 .
[0063] 3), 1g compound 3 and 1 g of 3-aminophenylacetylene were added to isopropanol. After reacting for 4-9 hours, the isopropanol was spin-dried, and then dichloromethane-methanol was used as the eluent, and the comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com