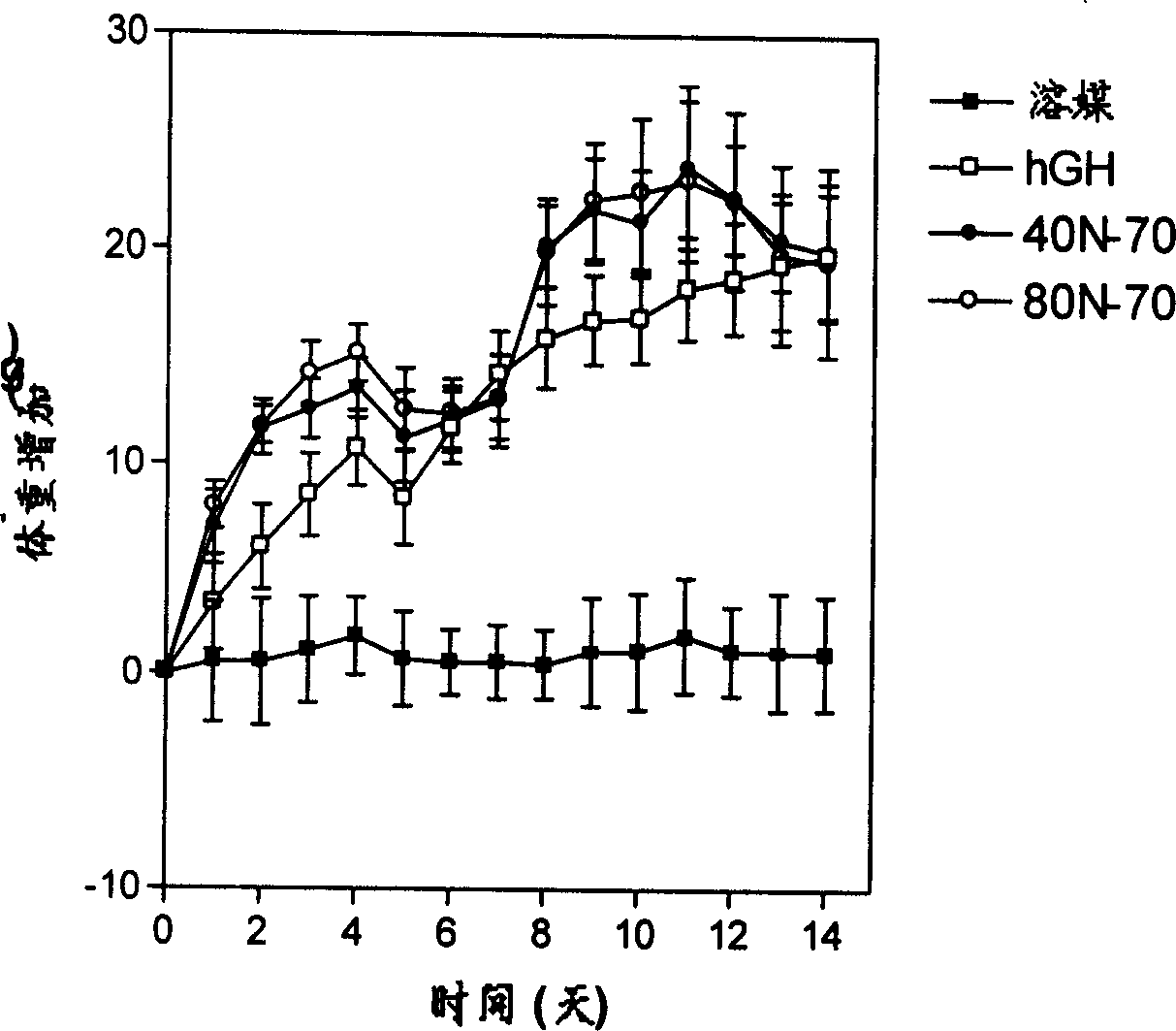
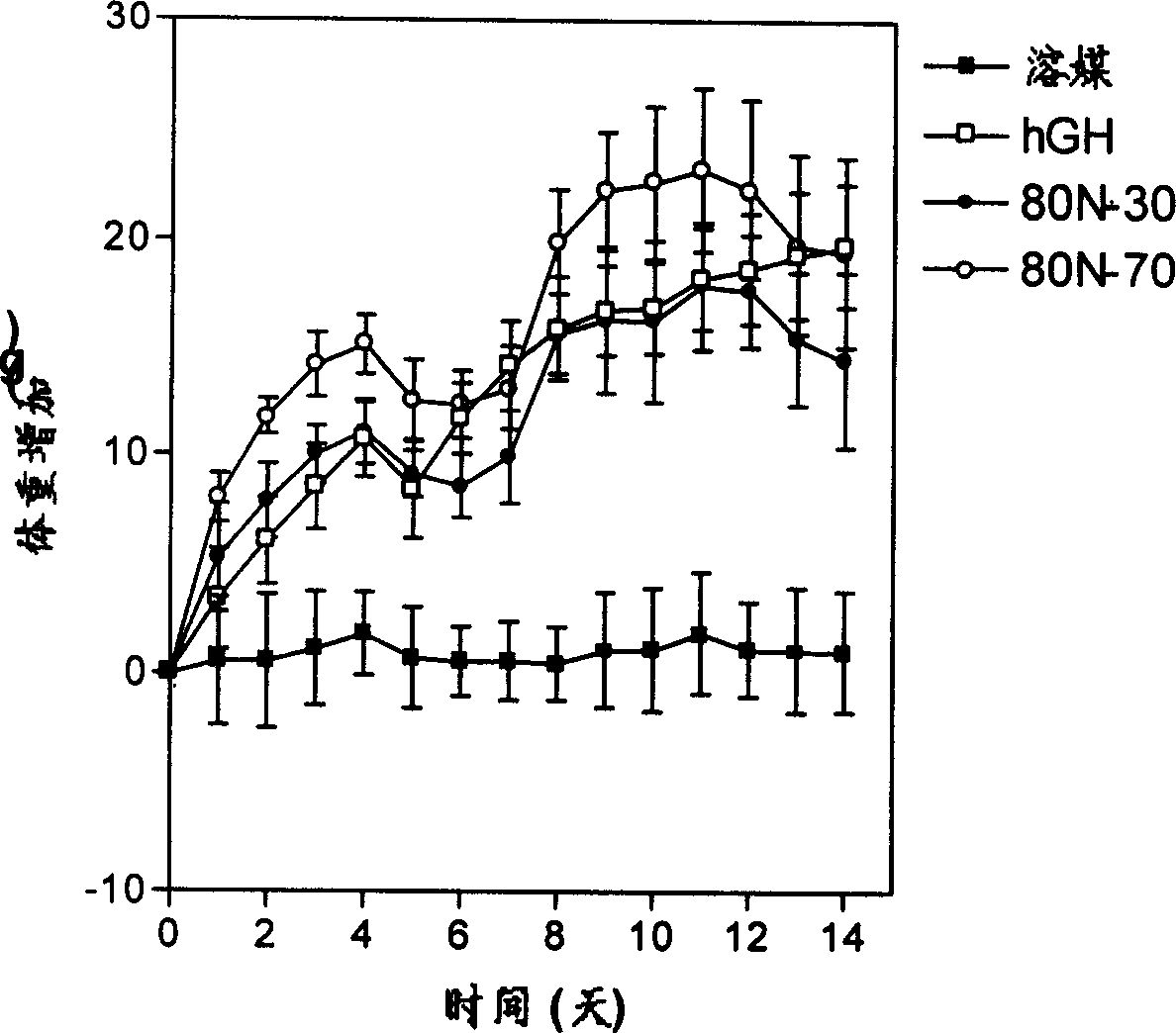
Long-acting growth hormone and medicine composition
A technology of growth hormone and conjugates, which is applied in the direction of drug combination, growth hormone, hormone peptide, etc., and can solve problems such as poor practicability, increased dosage, product inhomogeneity, process and quality control, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation of embodiment 1 high molecular weight bifurcated polyethylene glycol
[0064] In order to prolong the half-life of growth hormone, high molecular weight branched polyethylene glycol is more suitable. A method for synthesizing polyethylene glycol with a multi-branched molecular weight of 80,000 is described below, and this method can also be used to synthesize other polyethylene glycols with different molecular weights or shapes.
[0065] 11.8 mg of Fmoc-Lys(Fmoc)-OH (MW590, 20 μmol, AdvancedChemTech) was dissolved in 120 μl of dimethylformamide (DMF) containing 24 μmol of NHS (Sigma), and 3.8 μl of diisopropylcarbodiimide ( DIC, MW126, d=0.8, 24 μmol, Advanced ChemTech), the reaction was shaken at room temperature for two hours. Dissolve 68 mg of NH2-PEG-COOH (MW3400, 20 μmol, Shearwater) in 100 μl of dichloromethane (DCM), add 6.9 μl of diisopropylethylamine (DIPEA, MW129, d=0.74, 40 μmol, Sigma), and mix with the previous The partial reaction was mixed...
Embodiment 2
[0069] Embodiment 2 Somatotropin is coupled with 80kDa and 40kDa polyethylene glycol
[0070] Dissolve 234mg of growth hormone lyophilized powder in 4ml of 0.1M sodium phosphate, pH7.0. A HiTrap Sephadex G25 desalting column (2.5×15 cm, Pharmacia Biotech) was equilibrated with human 0.1 M sodium phosphate (pH 7.0), and the growth hormone sample was loaded, observed at 280 nm, and the first peak (18 ml) was collected. Measure the 280nm optical density and use the extinction coefficient of 0.68 to calculate the growth hormone concentration, the result is 280nm optical density 1.2, protein concentration 1.76mg / ml, total protein 32mg.
[0071] When coupling with 80kDa polyethylene glycol, weigh 60mg of activated 80kDamPEG 4 - NHS (0.7 μmol) was added to 1.1 ml of sodium phosphate (pH 7.0) solution containing 6 mg growth hormone (0.3 μmol), and the reaction was shaken at 4° C. overnight.
[0072] When coupling with 40kDa polyethylene glycol, weigh 60mg of activated 40kDamPEG 2 -...
Embodiment 380
[0073] Example 380kDa and 40kDa polyethylene glycol growth hormone conjugates are purified with Superdex75
[0074] The 80kDa and 40kDa polyethylene glycol somatotropin conjugates were purified by Superdex 75 chromatography column (1.5×40cm, Pharmacia Biotech). Equilibrate the chromatographic column with a solution of 150mM sodium chloride, 10mM sodium citrate, 0.2% Tween 20, and pH 6.0, and then load the sample. The sample volume is less than 5% of the column volume. Use 280nm to observe the protein peak and collect first eluting peak. A total of 35ml of 40kDa polyethylene glycol human growth hormone conjugate with a concentration of 0.16mg / ml was collected, with a total protein content of 5.6mg. A total of 28ml of 80kDa polyethylene glycol human growth hormone conjugate with a concentration of 0.08mg / ml was collected, with a total protein content of 2.6mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com