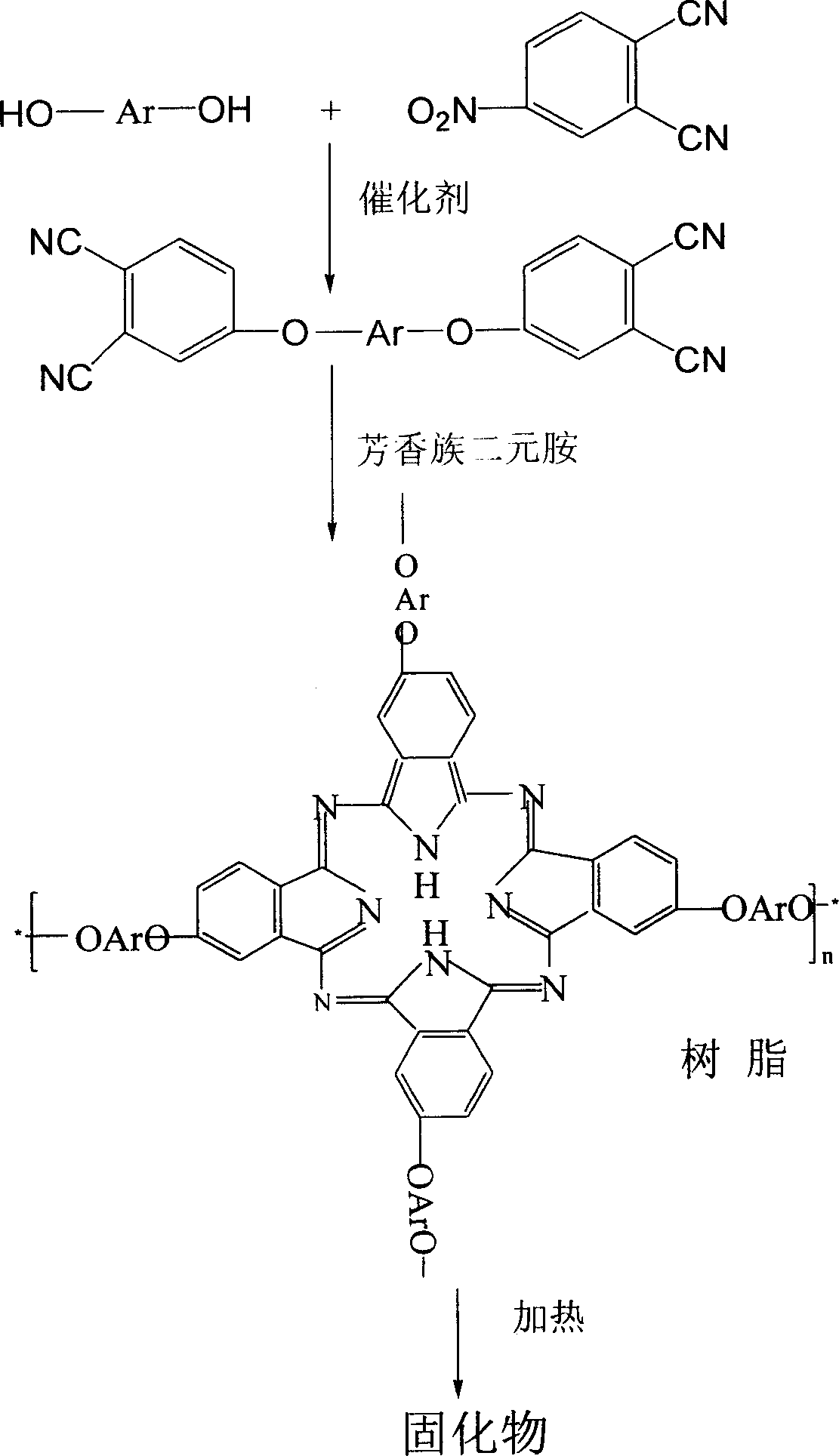
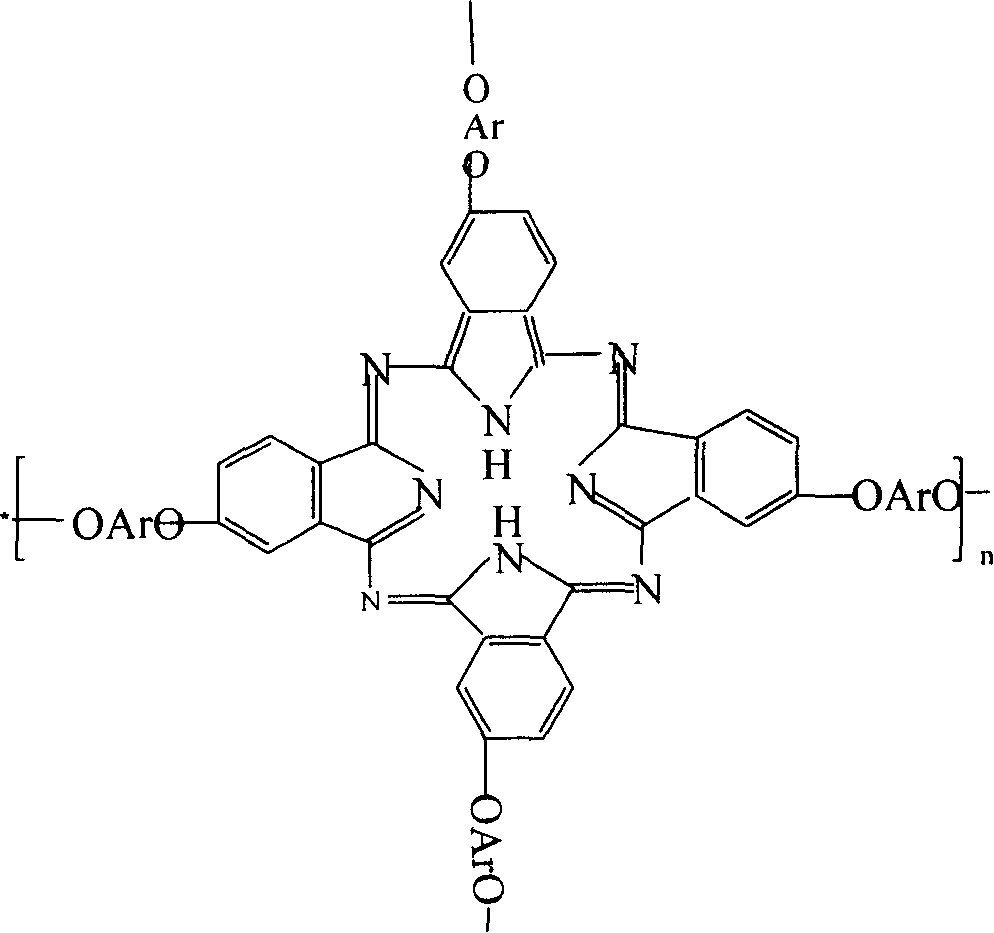
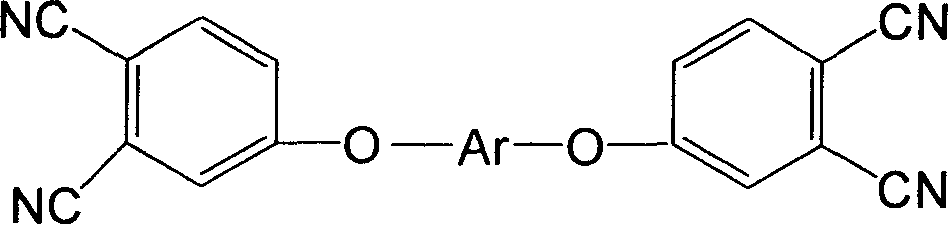Double end-group phthalonitrile, resin, condensate and its preparation method and uses
A technology of phthalonitrile resin and base phthalonitrile, which is applied in the field of polymer synthetic materials, can solve the problems of poor performance of cured products, complicated reaction mechanism, large creep at high temperature and the like, and achieves high thermal stability, Simple method and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 Preparation of double-terminated phthalonitrile monomer, phthalonitrile resin and cured product thereof of the present invention
[0050] 1. Synthesis of biphenyl-type double-terminated phthalonitrile monomer
[0051] 4-Nitrophthalonitrile 2mole
[0052] Biphenol 1mole
[0053] Anhydrous Potassium Carbonate 1.1mole
[0054] Add 30 moles of 4-nitrophthalonitrile, biphenol, anhydrous potassium carbonate, and solvent dimethylformamide into a four-necked bottle, feed nitrogen to replace the air therein, raise the temperature, and React at 85°C for 5 hours to stop the reaction, pour the reaction mixture into water to precipitate, add dilute hydrochloric acid to acidify, filter, wash with water three times, and dry in a vacuum oven at 80°C for 24 hours to obtain double-terminated biphenyl phthalonitrile .
[0055] After testing, the obtained biphenyl phthalonitrile monomer has a melting point of 227°C and a DSC curing peak temperature of 285°C.
[0056] 2. P...
Embodiment 2
[0064] Embodiment 2 Preparation of double-terminated phthalonitrile, phthalonitrile resin and cured product thereof of the present invention
[0065] 1. Synthesis of double-terminated biphenyl-type phthalonitrile
[0066] 4-Nitrophthalonitrile 2mole
[0067] Biphenol 1mole
[0068] Anhydrous sodium carbonate 1.5mole
[0069] Add 30 moles of 4-nitrophthalonitrile, biphenol, anhydrous sodium carbonate, and solvent dimethylacetamide into a four-necked bottle, feed nitrogen to replace the air therein, raise the temperature, and React at 80°C for 8 hours to stop the reaction, pour the reaction mixture into water to precipitate, add dilute hydrochloric acid to acidify, filter, wash with water three times, and dry in a vacuum oven at 80°C for 24 hours to obtain double-terminated biphenyl phthalonitrile.
[0070] After testing, the melting point of the biphenyl-type phthalonitrile monomer is 227°C, and the DSC curing peak temperature is 285°C;
[0071] 2. Preparation of phthalonit...
Embodiment 3
[0080] Example 3 Preparation of double-terminated phthalonitrile monomer, phthalonitrile resin and cured product thereof of the present invention
[0081] 1. Synthesis of double-terminated biphenyl-type phthalonitrile monomer
[0082] 4-Nitrophthalonitrile 2mole
[0083] Biphenol 1mole
[0084] Anhydrous potassium carbonate and anhydrous sodium carbonate (molar ratio 1 / 1) total 1.2mole
[0085] Add 30 moles of 4-nitrophthalonitrile, biphenol, anhydrous potassium carbonate and anhydrous sodium carbonate (molar ratio 1 / 1), and solvent dimethylacetamide into a four-necked bottle, and pass into Replace the air with nitrogen, raise the temperature, react at 90°C for 4 hours to stop the reaction, pour the reaction mixture into water to precipitate, add dilute hydrochloric acid to acidify, filter, wash with water three times, and dry in a vacuum oven at 80°C for 24 hours to obtain Double-terminated biphenyl phthalonitrile.
[0086] After testing, the melting point of the phthalon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
| gel time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com