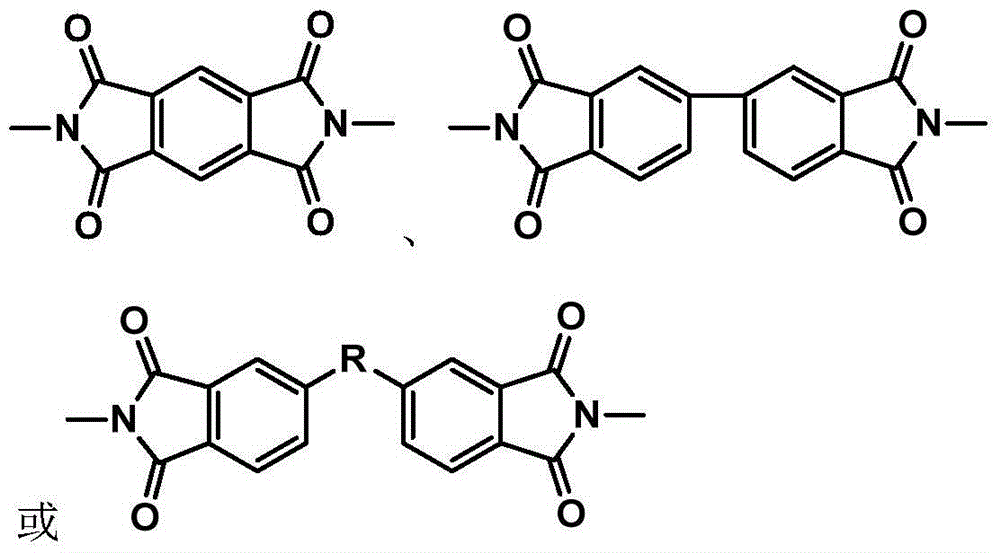
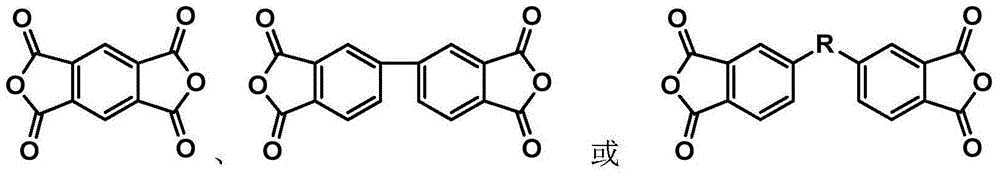
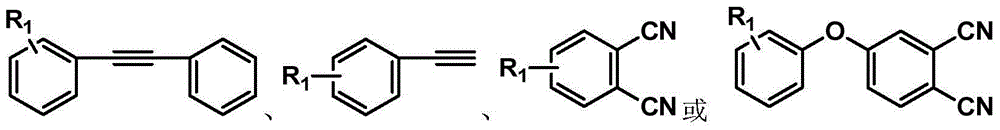Synthesis method of phthalonitrile and arylacetylene-terminated aromatic imide
A technology of phthalonitrile and arylimide, applied in the field of synthesis of arylimide monomers, can solve the problems of low processing efficiency, difficult processing, slowness and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1.1. Synthesis of one-component system phthalonitrile and phenylacetylene-terminated imide monomer a1
[0070] 1.1.1. Synthesis of Intermediate 1
[0071] Put 3.10g of 4,4'-biphenyl ether dianhydride (ODPA) into 7ml of NMP, and after ODPA is completely dissolved, slowly add compound B (the structural formula of compound B) into the ODPA solution dropwise at room temperature The NMP solution (the mol ratio of ODPA and compound B is 1:1, V NMP =(total solid mass (g)*2)mL), after the dropwise addition was completed, react at room temperature for 6h. Then add acetic anhydride and pyridine (ODPA: acetic anhydride: pyridine molar ratio is 1:6:6) into the reaction solution, after the addition is completed, the temperature is raised to 100° C. for 3 h. Then lower the temperature, precipitate with anhydrous methanol, filter, collect the filtrate, and distill the filtrate under reduced pressure to remove methanol. Afterwards, the obtained concentrated solution was washed with ...
Embodiment 2
[0085] 2.1. Synthesis of one-component system phthalonitrile and phenylacetylene-terminated imide monomer a2
[0086] 2.1.1. Synthesis of intermediate 1
[0087] With the pyromellitic anhydride (PMDA) of 2.18g, drop in the DMF of 22ml, after treating that PMDA dissolves completely, in the PMDA solution, slowly add the DMF solution that dissolves compound B (the mol of PMDA and compound B) in the PMDA solution at room temperature The ratio is 1:1.2, V DMF =(Total solid mass (g)*10)mL) After the dropwise addition was completed, react at room temperature for 12 hours. Then add acetic anhydride and pyridine (the molar ratio of PMDA: acetic anhydride: pyridine is 1:6:6) to the reaction liquid, and after the addition is completed, the temperature is raised to 120° C. for 6 h. Then lower the temperature, precipitate with anhydrous methanol, filter, collect the filtrate, and distill the filtrate under reduced pressure to remove methanol. Afterwards, the obtained solid was washed wi...
Embodiment 3
[0101] 3.1. Synthesis of one-component system phthalonitrile and phenylacetylene-terminated imide monomer a3
[0102] 3.1.1. Synthesis of intermediate 1
[0103] Put 2.94g of 3,3',4,4'-biphenyltetracarboxylic anhydride (BPDA) into 10ml of DMAc, and after the BPDA is completely dissolved, slowly add the dissolved compound B into the BPDA solution at room temperature (the structural formula of compound B is as shown in the following synthesis process) DMAc solution (the mol ratio of BPDA and compound B is 1:1.1, V DMAC =(total solid mass (g)*3.3)mL) After the dropwise addition, react at room temperature for 9h. Then add acetic anhydride and pyridine (the molar ratio of BPDA: acetic anhydride: pyridine is 1:6:6) to the reaction solution, and after the addition is completed, the temperature is raised to 110° C. for 4.5 hours. Then lower the temperature, precipitate with anhydrous methanol, filter, collect the filtrate, and distill the filtrate under reduced pressure to remove me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
| storage modulus | aaaaa | aaaaa |
| modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com