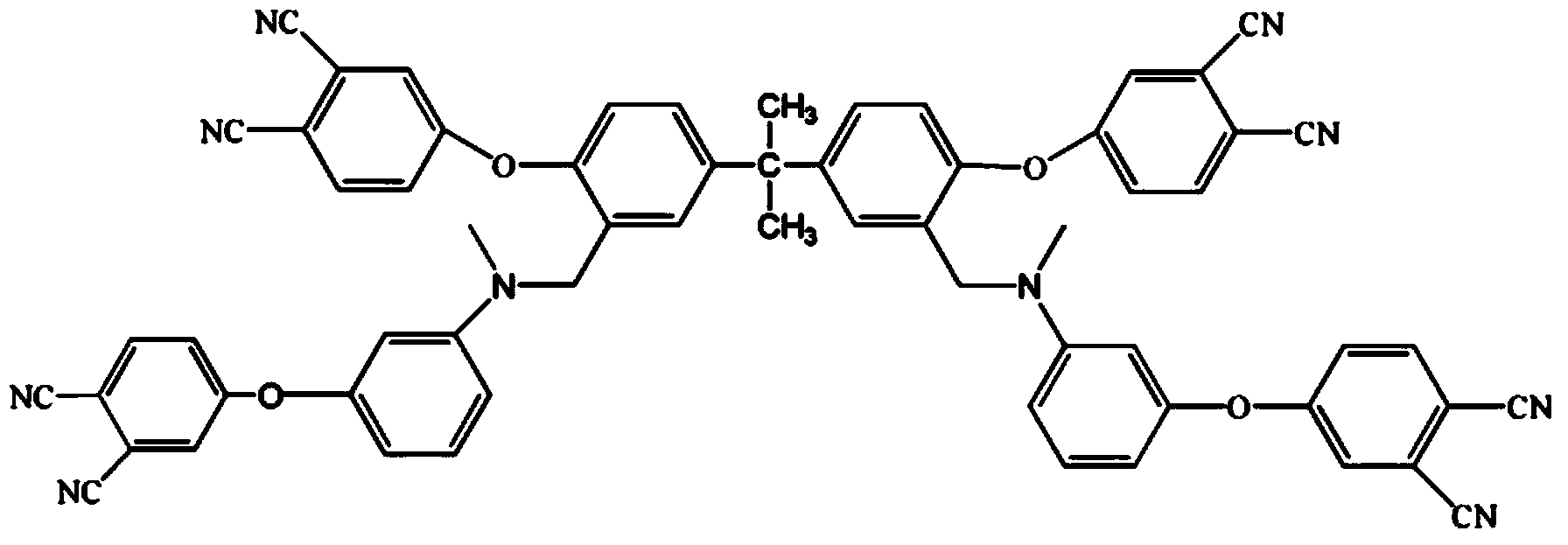
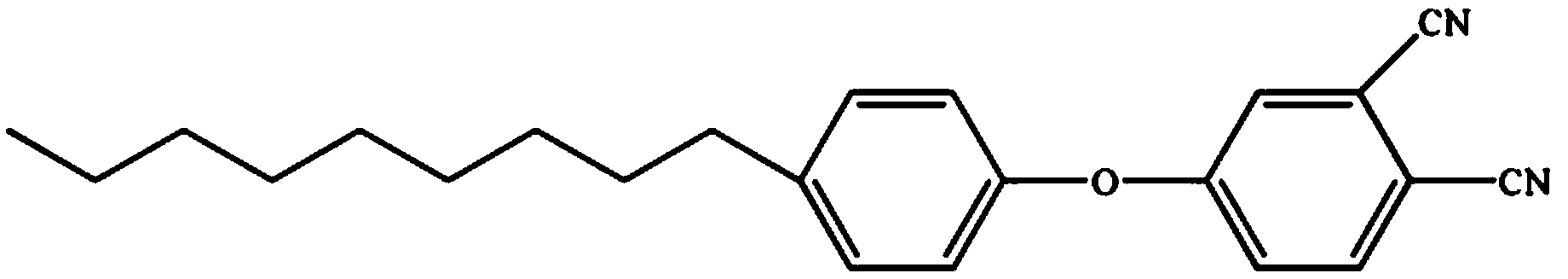
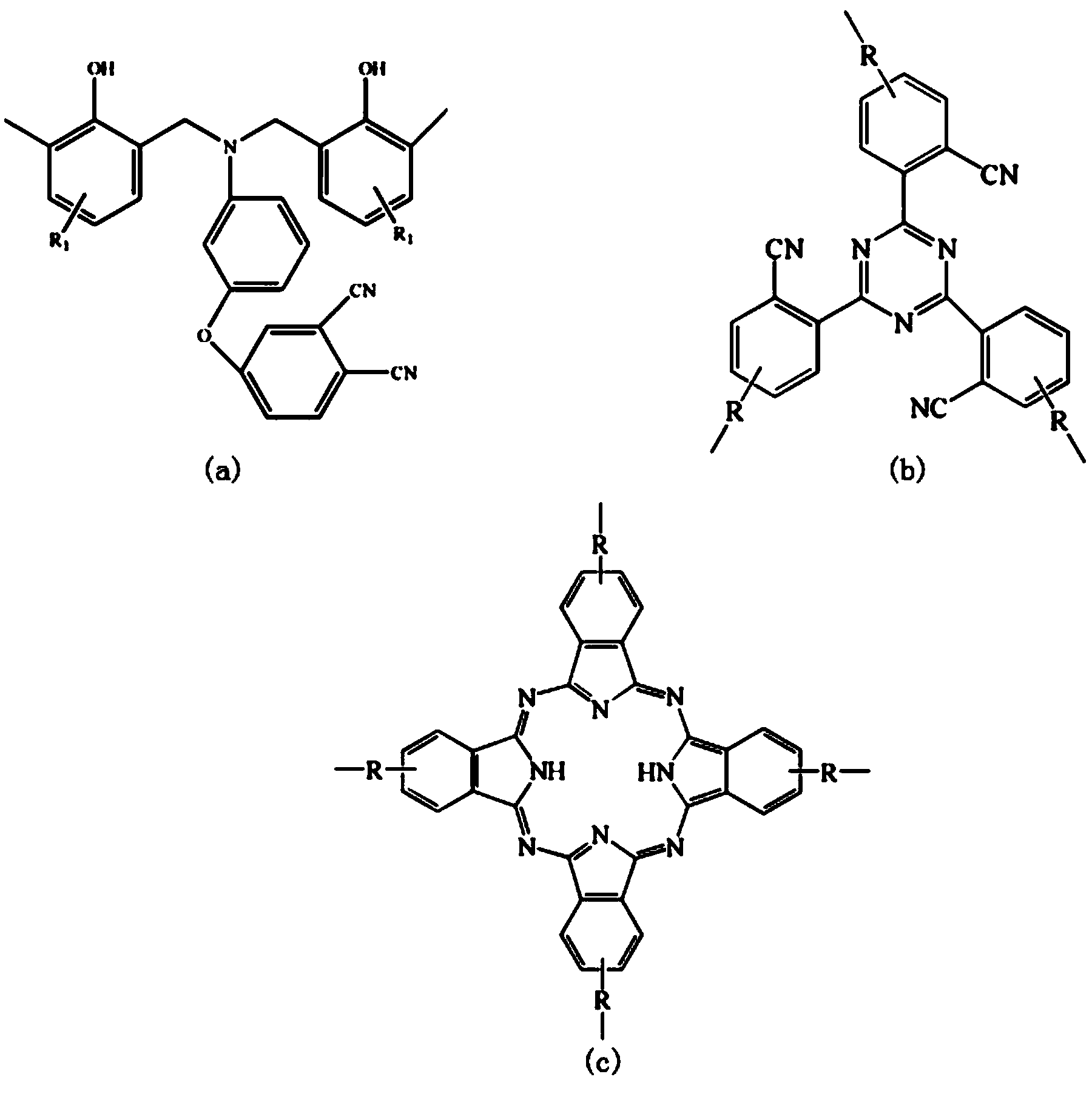
Low-viscosity cyano resin monomer and polymer, and preparation method thereof
A nitrile-based resin and low-viscosity technology, which is applied in the field of low-viscosity nitrile-based resin monomers and polymers and their preparation, can solve the problems that heat resistance and flame retardancy cannot meet material performance requirements, low crosslinking density, and the like, Achieve the effect of good molecular structure design, low water absorption and excellent curing reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of low-viscosity nitrile resin monomer:
[0041] Add 1g of p-nonylphenol-type phthalonitrile monomer into the reaction vessel, heat to 60°C, then add 9g of tetraphthalonitrile monomer containing benzoxazine ring and stir for 30 minutes; then cool to room temperature.
[0042] The low-viscosity nitrile-based resin monomer thus prepared has a viscosity of 20 Pa.s at room temperature and a curing peak temperature of 223°C.
[0043] (2) Preparation of low-viscosity nitrile-based resin polymer:
[0044] Heating the previously prepared low-viscosity nitrile-based resin monomer to 100-180°C for 1-5 hours to react, and then heat-treating at 240°C for 3-5 hours to obtain the nitrile-based resin polymer.
[0045] The Nitrile Polymer 1010 cm -1 (phthalocyanine ring); 1360 cm -1 and 1520 cm -1 (triazine ring); 1246, 1208 cm -1 (Ar-O-Ar); 832, 782, 729 cm -1The characteristic absorption peaks appeared at the (benzene ring). The low-viscosity nitrile-based resi...
Embodiment 2
[0047] (2) Preparation of low-viscosity nitrile-based resin monomer:
[0048] Add 3g of p-nonylphenol-type phthalonitrile monomer into the reaction vessel, heat to 60°C, then add 7g of tetraphthalonitrile monomer containing benzoxazine ring and stir for 30 minutes; then cool to room temperature.
[0049] The low-viscosity nitrile-based resin monomer thus prepared has a viscosity of 10 Pa.s at room temperature and a curing peak temperature of 228°C.
[0050] (2) Preparation of low-viscosity nitrile-based resin polymer:
[0051] Heating the previously prepared low-viscosity nitrile-based resin monomer to 100-180°C for 1-5 hours to react, and then heat-treating at 240°C for 3-5 hours to obtain a low-viscosity nitrile-based resin polymer.
[0052] The Low Viscosity Nitrile Polymer 1010 cm -1 (phthalocyanine ring); 1360 cm -1 and 1520 cm -1 (triazine ring); 1246, 1208 cm -1 (Ar-O-Ar); 832, 782, 729 cm -1 The characteristic absorption peaks appeared at the (benzene ring). Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com