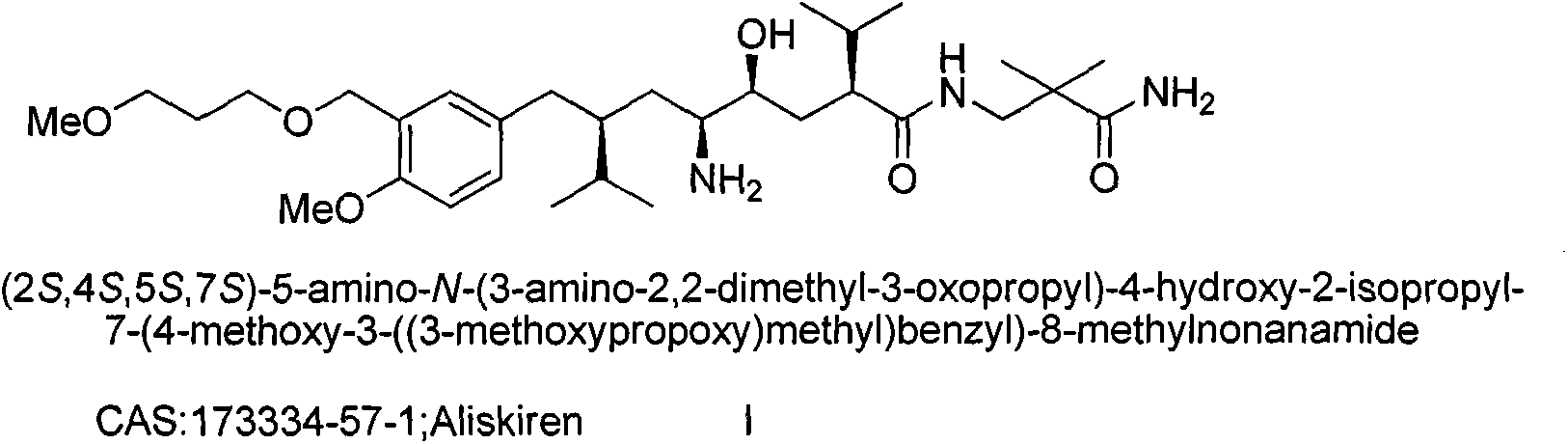
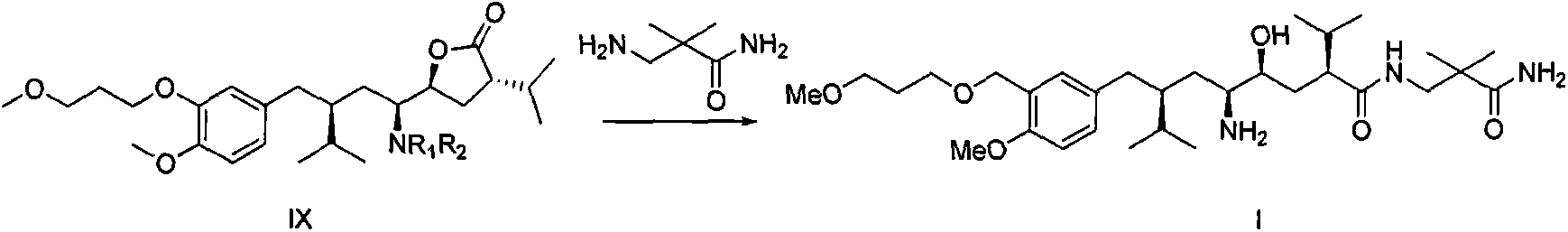
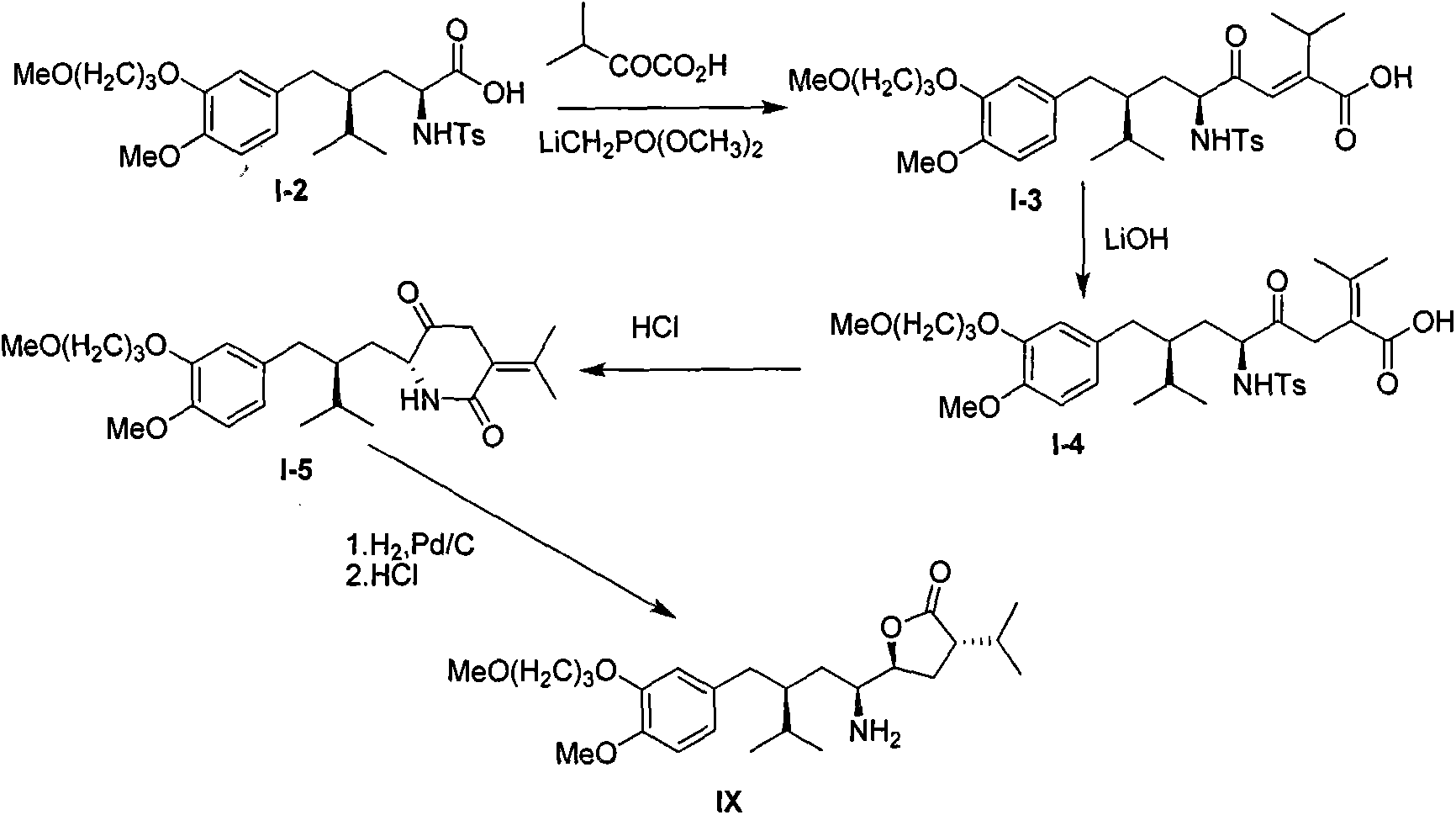
Preparation method for intermediate 3-isopropylfuranone derivatives of blood-pressure-reducing medicine aliskiren
A technology of propylfuranone and an intermediate, which is applied in the field of pharmaceutical preparation, can solve the problems of complex synthesis and long steps of the intermediate compound V-3, and achieves the effects of low cost, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] In order to fully illustrate the essence of the patent of the present invention, preparation ideas and ideas, verify the preparation method described in the present invention in the following examples, these examples are only for illustration and special case representative, should not be interpreted or understood as protection of the present invention limit. Embodiment 1: the preparation of intermediate:
[0055] Step 1: Preparation of (2S, 4S)-2-amino-4-(4-methoxy-3-(3-methoxypropoxy)benzyl)-5-methylhexanoic acid (II) :
[0056] according to Helv. Chim. Acta , 2003, 86, synthesized by the method of 2848-2868.
example 2
[0058] The second step: (2S, 4S)-2-((N,N-dibenzyl)amino)-4-(4-methoxy-3-(3-methoxypropoxy)benzyl)- The preparation of 5-methylhexanoic acid benzyl ester (III):
[0059]
[0060] Add (2S, 4S)-2-amino-4-(4-methoxy-3-(3-methoxypropoxy) successively to a 500mL three-necked round-bottomed flask equipped with a spherical condenser, mechanical stirring and a thermometer ) benzyl)-5-methylhexanoic acid (formula compound II) (19.0g, 53.8mmol), potassium carbonate (35g, 253.6mmol), ethanol 300mL, water 100m, benzyl chloride (27.0g, 213.3mmol), Stir and heat to reflux, react for 8 hours, and TLC detects that the reaction raw materials are completely reacted. Ethanol was evaporated under reduced pressure at 50°C, excess benzyl chloride was evaporated under reduced pressure at 90°C, 100 mL of deionized water was added, ethyl acetate (100 mL×3) was added for extraction, and the combined organic phases were sequentially washed with deionized water (100 mL) and saturated salt Washed with...
example 3
[0063] The third step: (3S, 5S)-1-chloro-3-((N,N-dibenzyl)amino)-5-(4-methoxy-3-(3'-methoxypropoxy) ) benzyl)-6-methyl-2-heptanone (formula compound IV) preparation:
[0064]
[0065] Under nitrogen protection, add 9.0 g (2S, 4S)-2-((N, N'-dibenzyl)amino)-4-(4-methoxy-3- (3-methoxypropoxy)benzyl)-5-methylhexanoic acid benzyl ester (compound of formula III) (9.0g14.4mmol), bromochloromethane (8.2g63.6mmol) and 90mlTHF, dry ice / acetone cooling To -78°C, n-butyllithium (45ml2.5mol / L112.5mmol) was slowly added dropwise, and reacted for 4 hours, and the reaction raw materials were detected by HPLC to complete the reaction. Add saturated ammonium chloride aqueous solution (80ml) to quench the reaction, recover the solvent under reduced pressure, add dichloromethane (150ml×3) for extraction, wash with saturated brine, recover the solvent under reduced pressure, and the resulting residue is purified by a short silica gel column and concentrated to obtain Compound represented by f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com