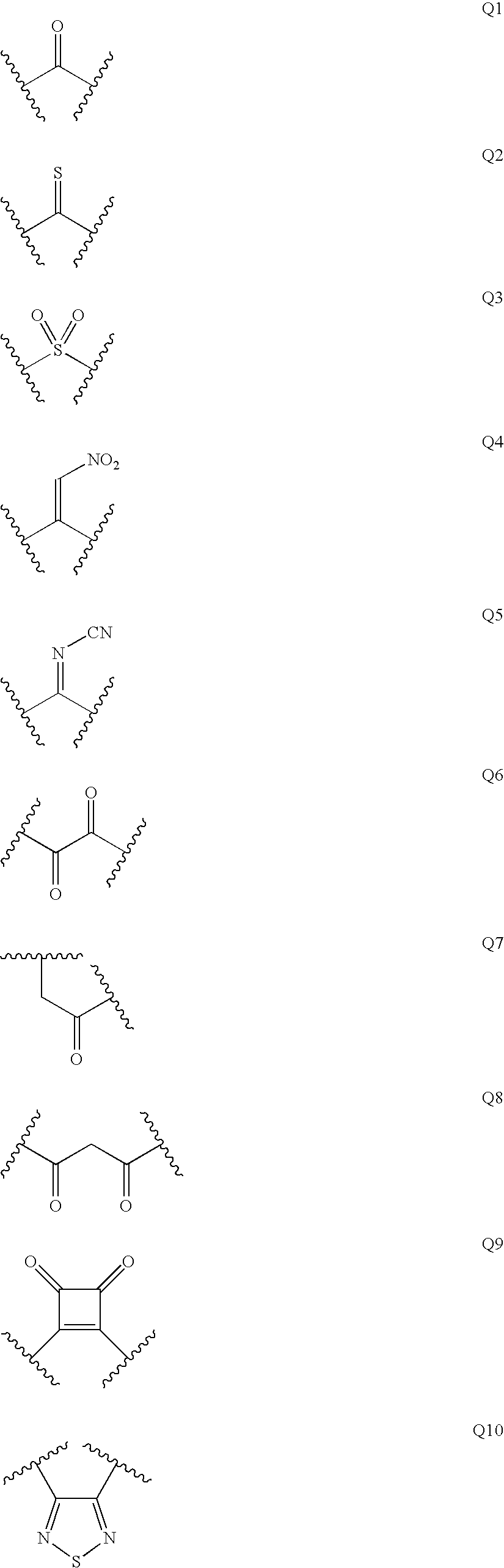
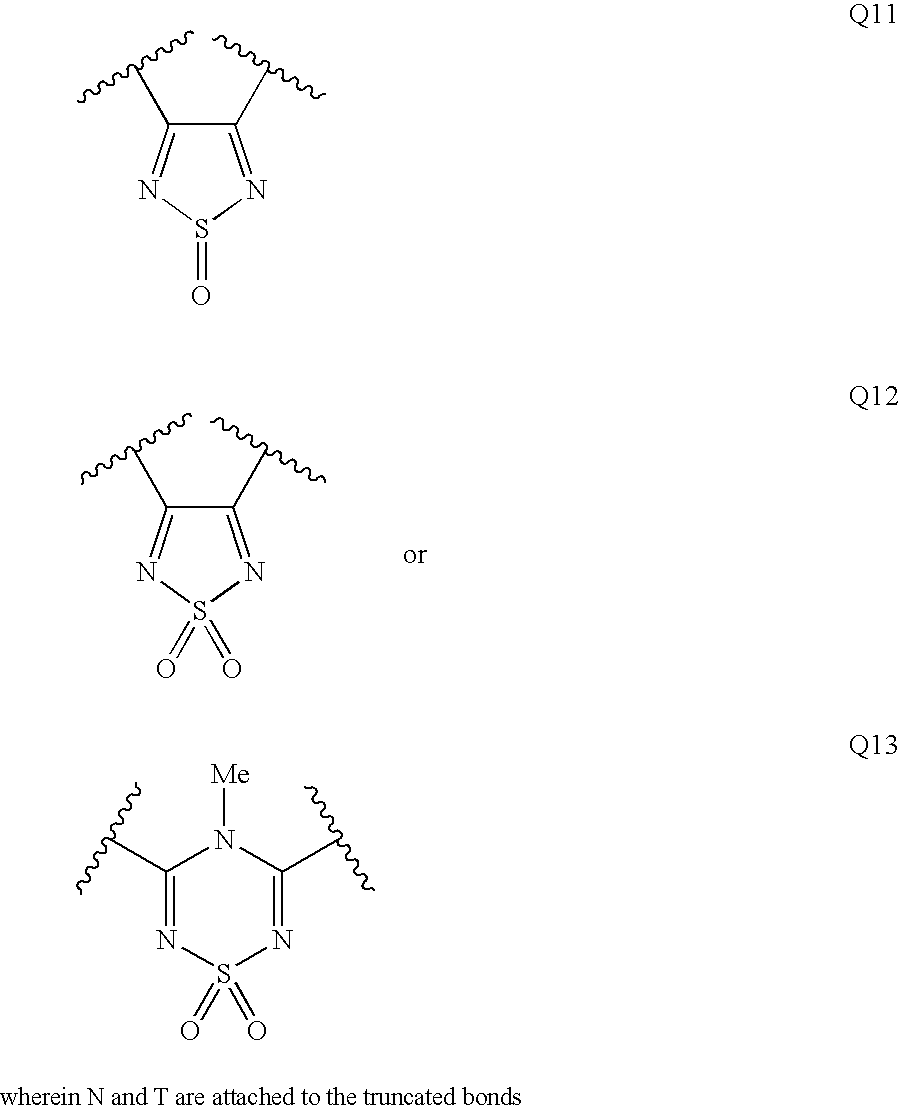
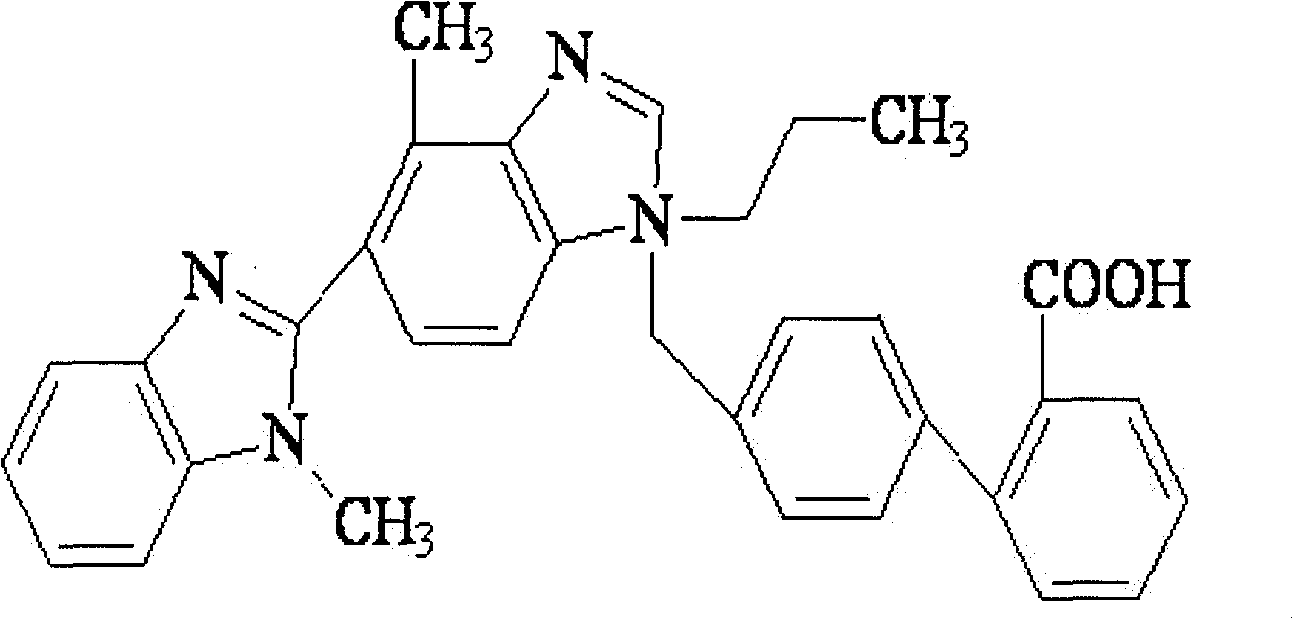
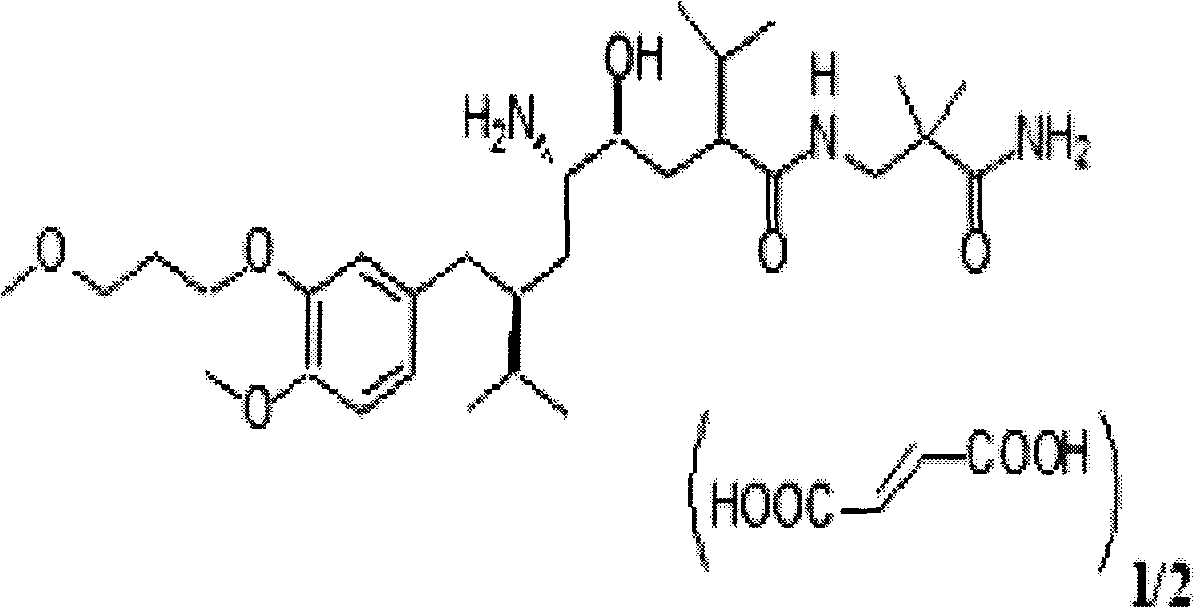
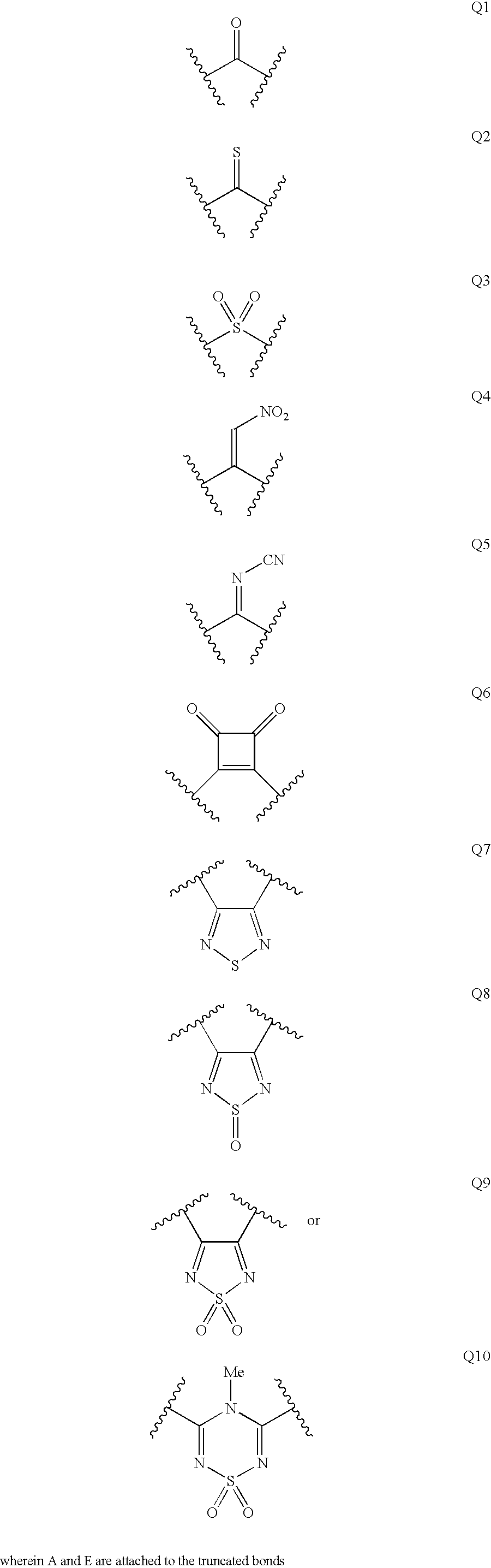
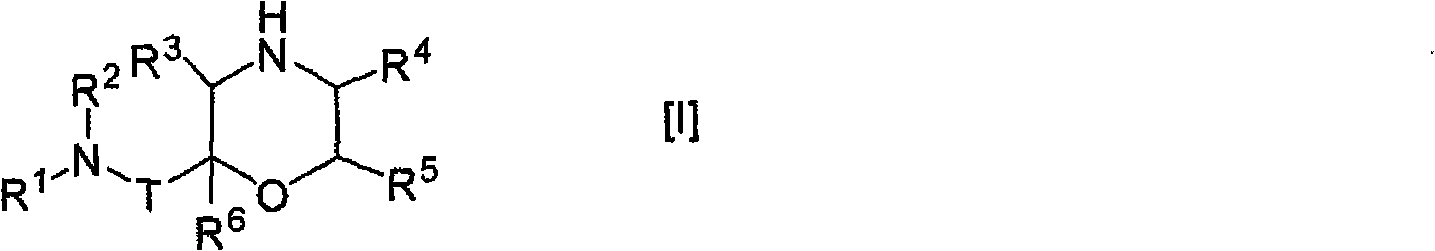Patents
Literature
107 results about "Renin inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Renin inhibitors are a group of pharmaceutical drugs used primarily in treatment of essential hypertension (high blood pressure). These drugs inhibit the first and rate-limiting step of the renin–angiotensin–aldosterone system (RAAS), namely the conversion of angiotensinogen to angiotensin I. This leads to a totality in absence of Angiotensin II based on the rationale that renin only acts to inhibit this step unlike Angiotensin Converting Enzyme which is also involved in other biochemical reactions. Since the 1970s, scientists have been trying to develop potent inhibitors with acceptable oral bioavailability. The process was difficult and took about three decades. The first and second generations faced problems such as poor bioavailability and lack of potency. Finally, the third generation was discovered. These compounds were nonpeptidic renin inhibitors, had acceptable oral bioavailability and were potent enough for clinical use. The first drug in this class was aliskiren, which received a marketing approval in 2007. As of January 2012, it is the only renin inhibitor on the market.
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Treatment of Prevention of Unscheduled Bleeding in Women on Progestogen Containing Medication
InactiveUS20090197843A1Increased chronotropismIncreased inotropismBiocidePeptide/protein ingredientsPhysiologyProgestogen
The present invention relates to a method of treating or preventing unscheduled bleeding in women, the unscheduled bleeding being the result of repeated administration of a hormonal composition that contains a progestogen, wherein the method includes the administration of an effective amount of Renin Angiotensin System (RAS) suppressor selected from angiotensin converting enzyme inhibitors; angiotensin II receptor antagonists; renin inhibitors and combinations thereof. Other aspects of the invention relate to a pharmaceutical composition containing a RAS suppressor and a progestogen and to a pharmaceutical kit having a plurality of dosage units, wherein at least one dosage unit contains a progestogen; at least one dosage unit contains an estrogen; and at least one dosage unit contains a RAS suppressor.
Owner:PANTARHEI BIOSCI
Methods for breast cancer screening and treatment
InactiveUS20100029734A1Decreasing Ang II-induced cell proliferationPromote growthBiocideDisease diagnosisBreast cancer screeningACE Inhibitor Fetopathy
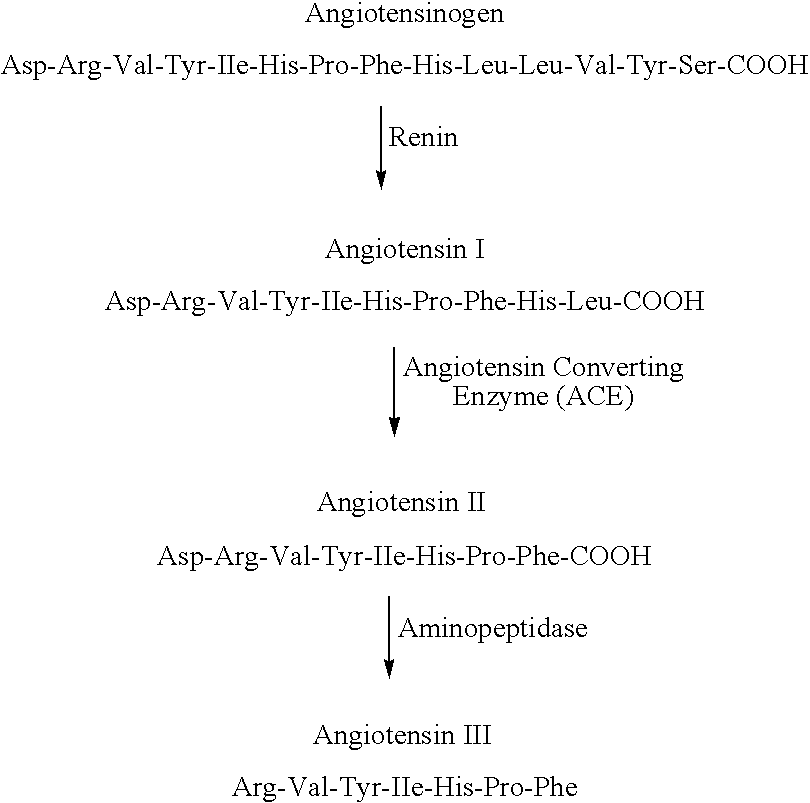
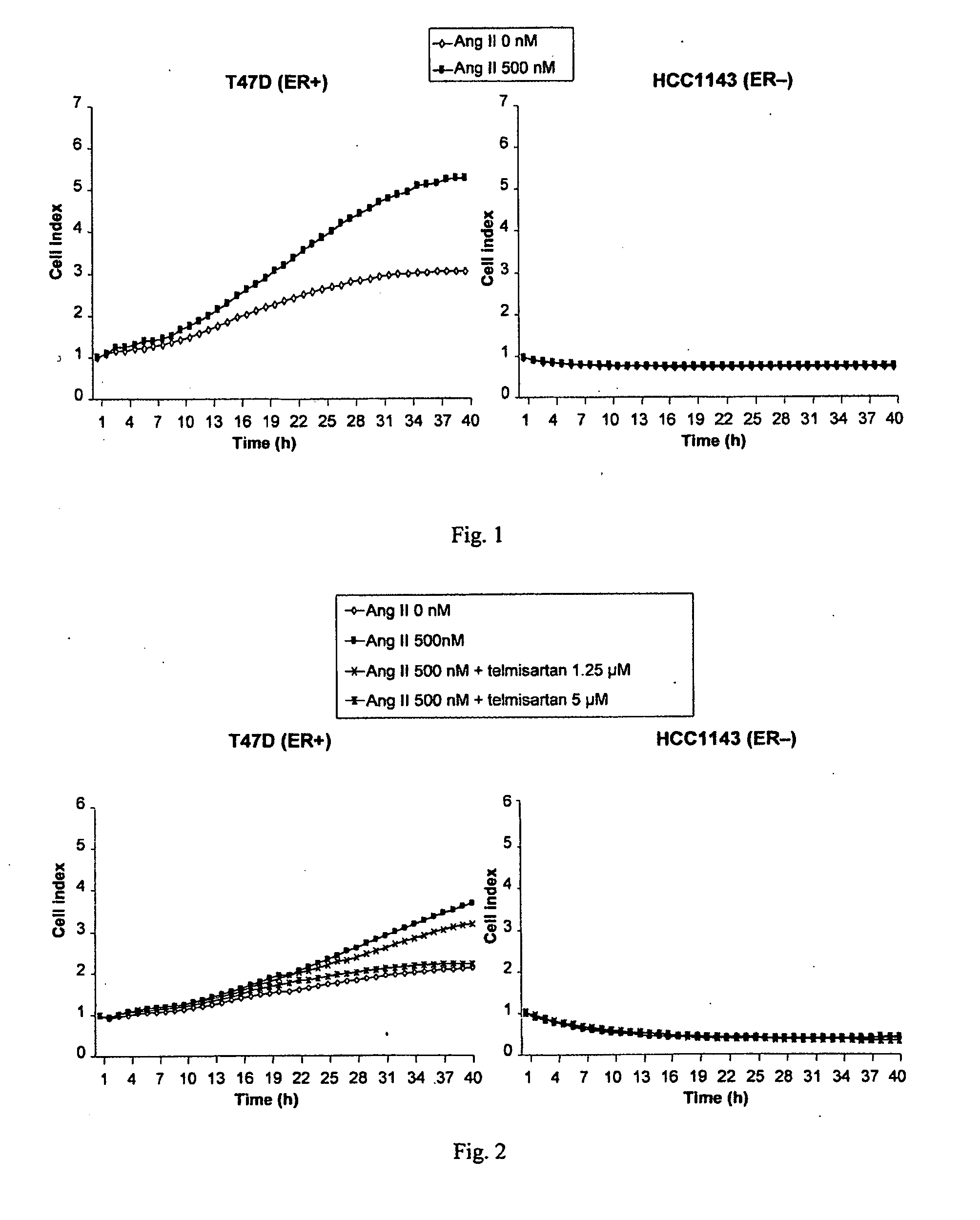
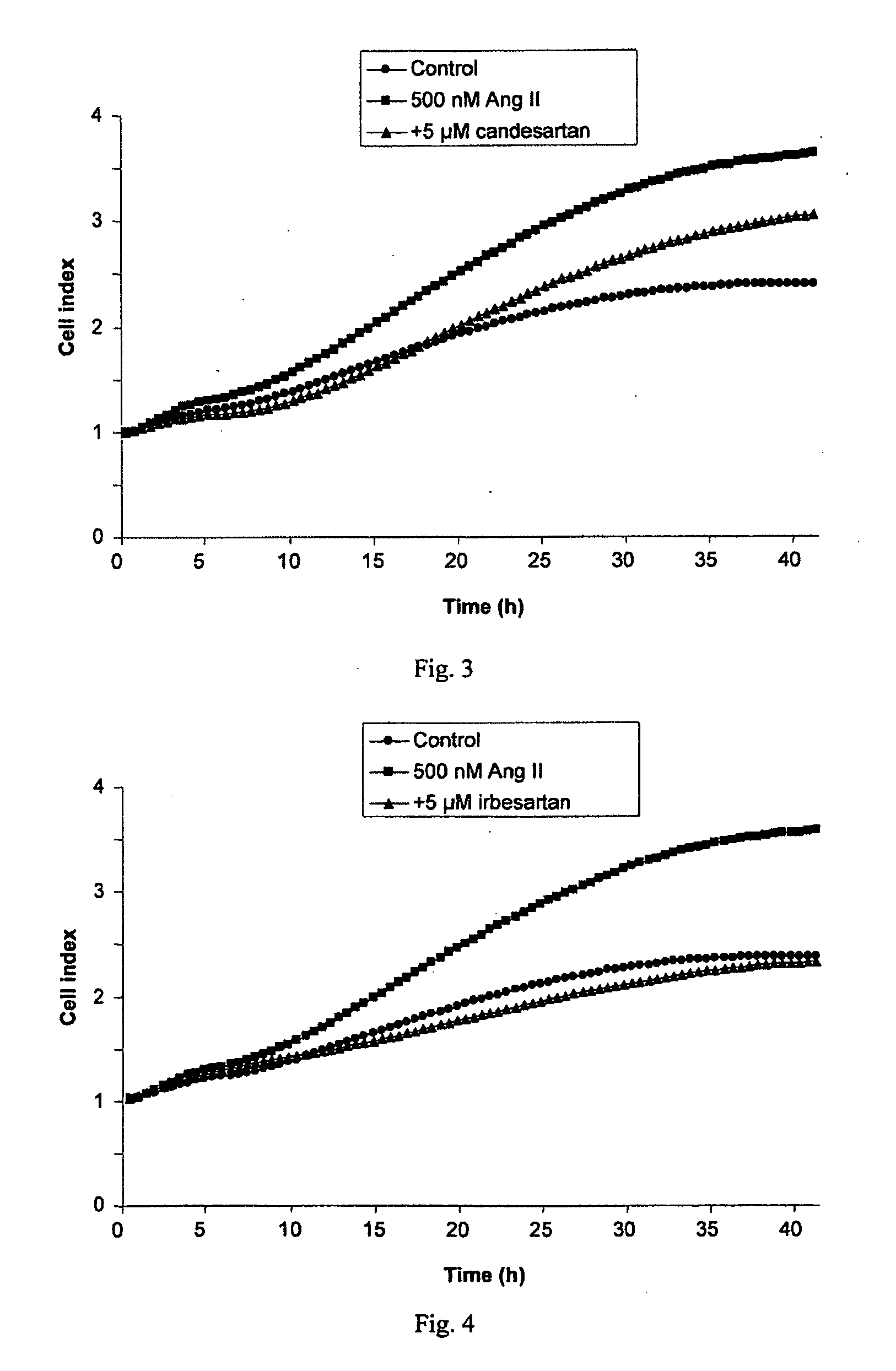
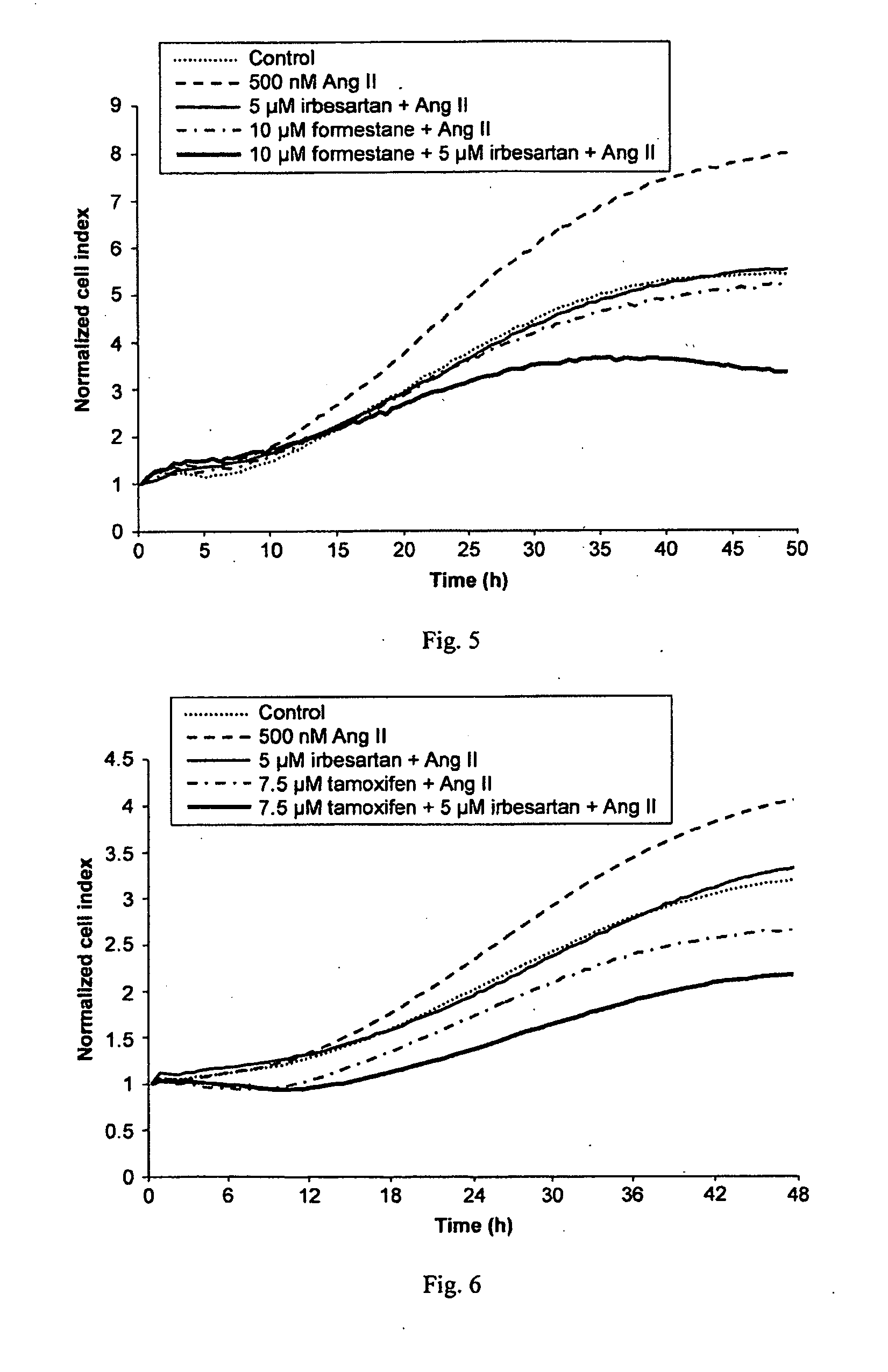
A method for selecting a breast cancer patient for therapy with an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor, comprises (a) determining whether the cancer comprises a tumor that is estrogen receptor positive (ER+) and (b) selecting the patient for such therapy only if the cancer is determined to comprise an ER+ tumor. A method for treating breast cancer in a patient further comprises (c) administering to the patient, if so selected, an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor. A method for treating a breast tumor in a patient having SERM-resistant ER+ breast cancer comprises administering to the patient an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor. A therapeutic combination useful in treatment of a breast tumor comprises an agent that reduces production of angiotensin II, for example an ACE inhibitor or renin inhibitor, and a second agent that comprises (a) an aromatase inhibitor or (b) an estrogen receptor modulator or antagonist.
Owner:ORE PHARMA
Combination of Organic Compounds
The invention relates to a combination, such as a combined preparation or a pharmaceutical composition, respectively comprisinga renin inhibitor, or a pharmaceutically acceptable salt thereof, andat least one therapeutic agent selected from the group consisting of(a) a specific anti-dyslipidemic agent and(b) a specific anti-obesity agentor, in each case, a pharmaceutically acceptable salt thereof.
Owner:WEBB RANDY LEE
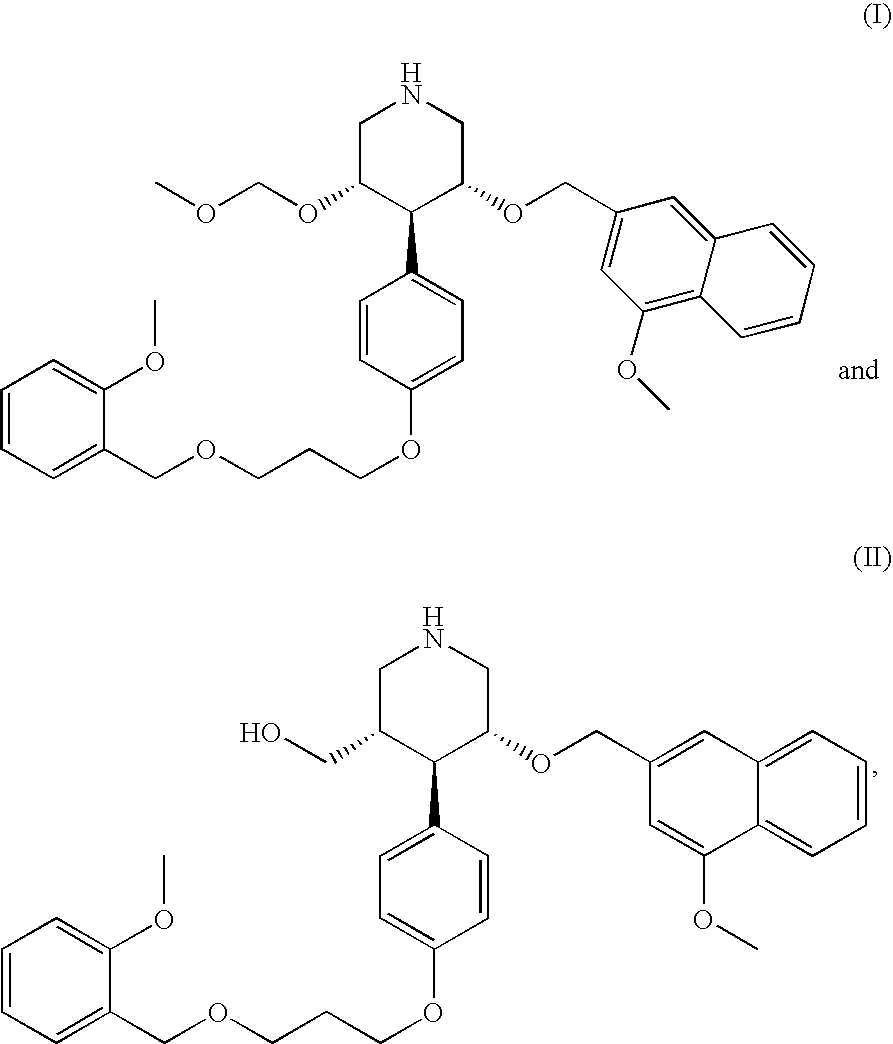
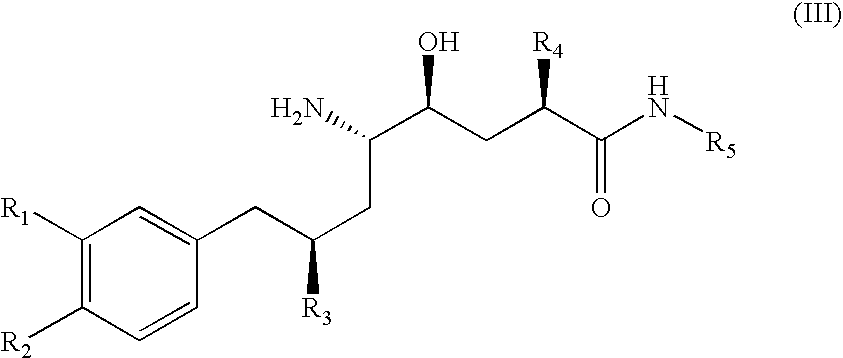
Renin inhibitors
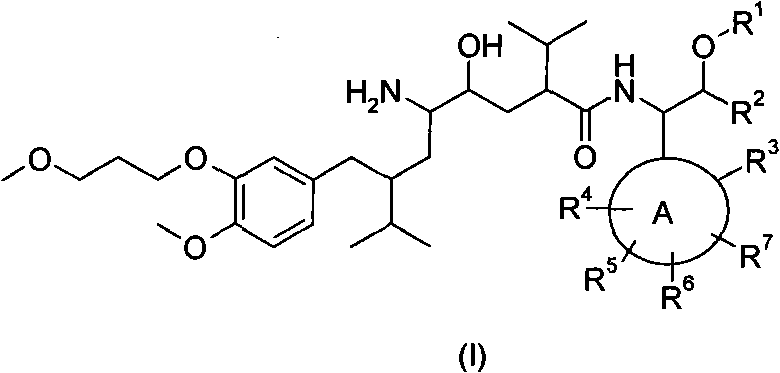
Disclosed are aspartic protease inhibitors represented by the following structural formula: and pharmaceutically acceptable salts thereof. These compounds are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. The present invention is also directed to pharmaceutical compositions comprising a compound described herein or enantiomers, diastereomers, or salts thereof and a pharmaceutically acceptable carrier or excipient.
Owner:VITAE PHARMA INC
Piperidine derivatives as renin inhibitors
The present invention is directed to aspartic protease inhibitors represented by the following structural formula; or a pharmaceutically acceptable salt thereof. The present invention is also directed to pharmaceutical compositions comprising the aspartic protease inhibitors of Structural Formula (I). Methods of antagonizing one or more aspartic proteases in a subject in need thereof, and methods for treating an aspartic protease mediated disorder in a subject using these aspartic protease inhibitors are also disclosed.
Owner:VITAE PHARMA INC
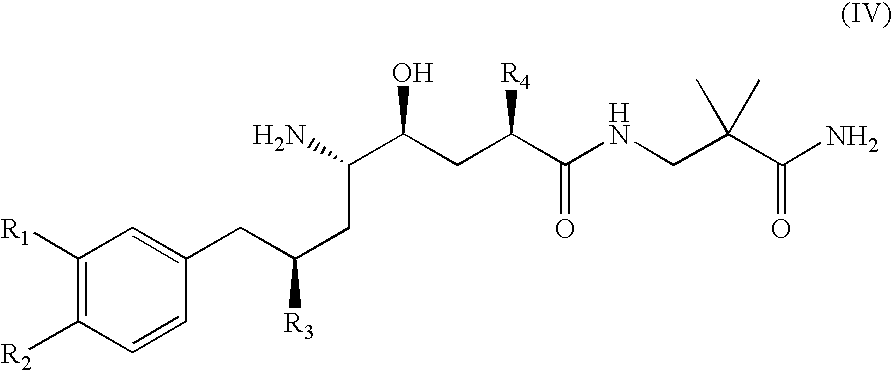
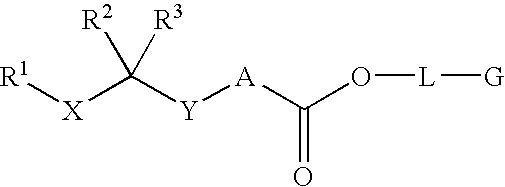
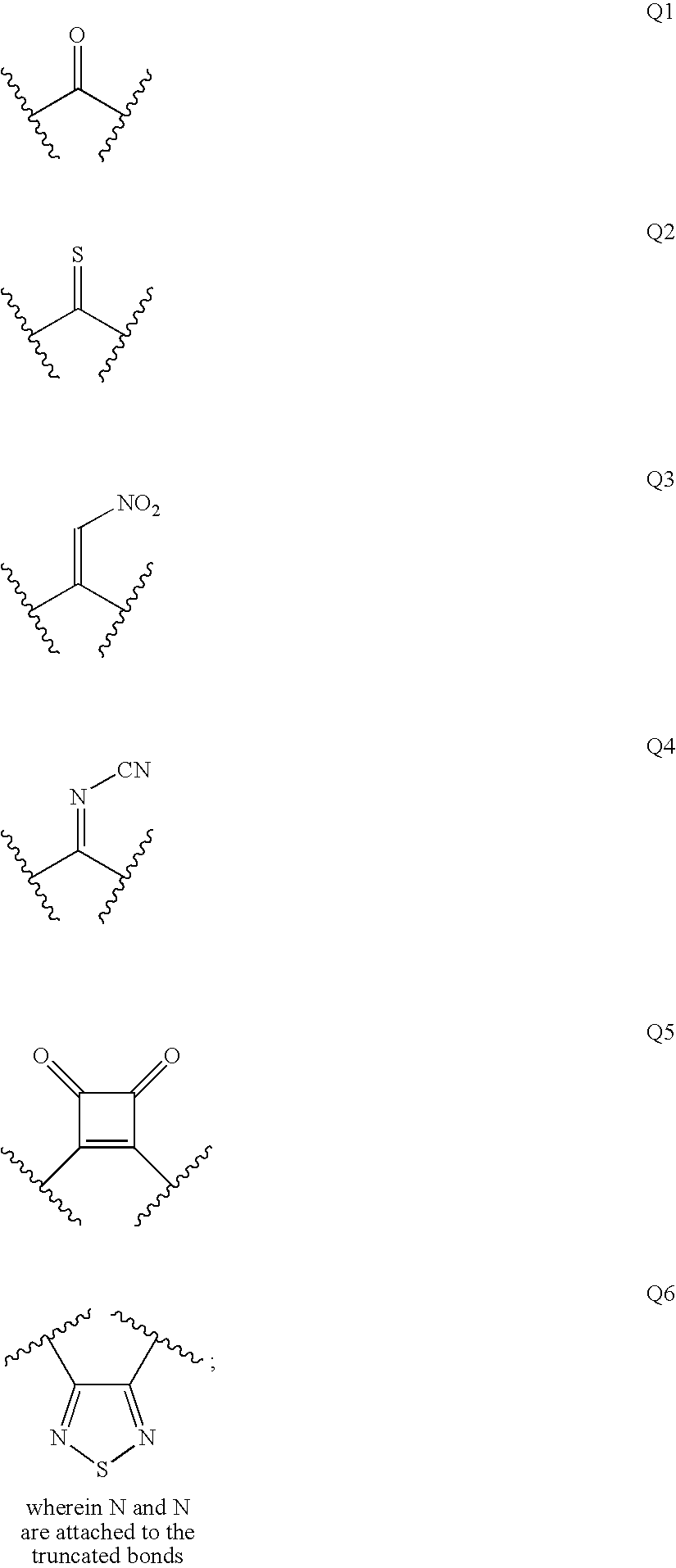
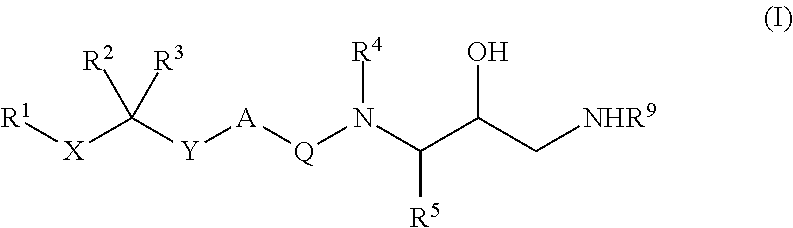
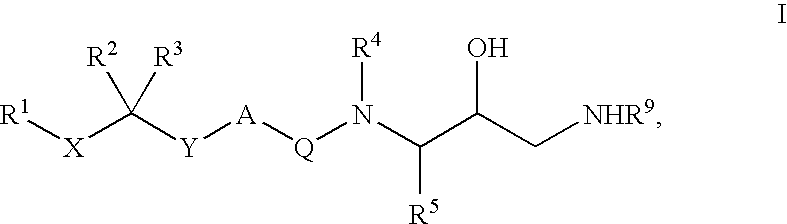
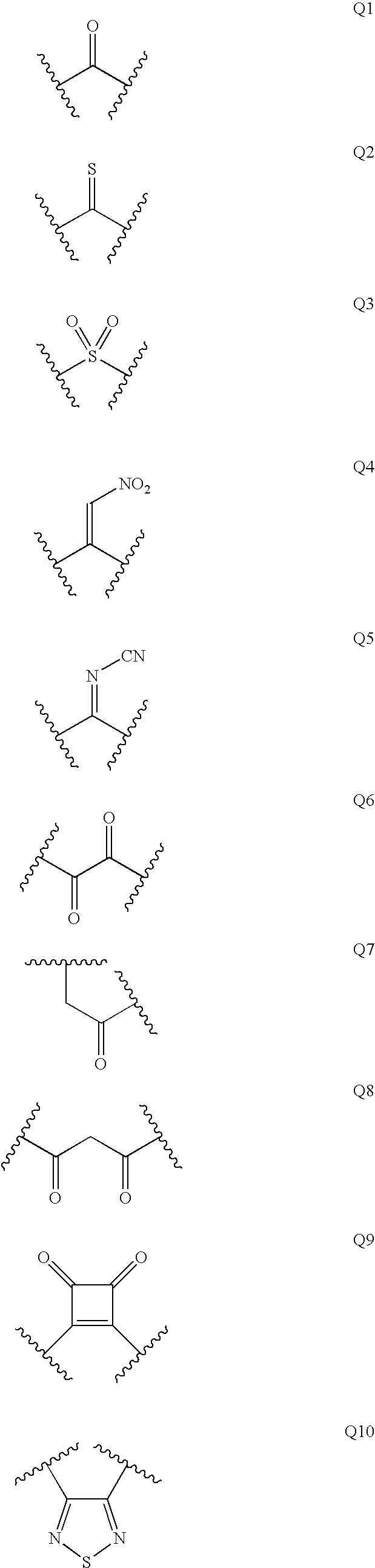
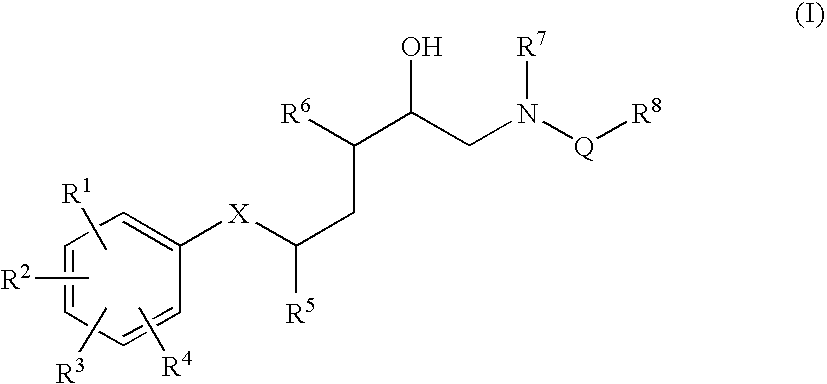
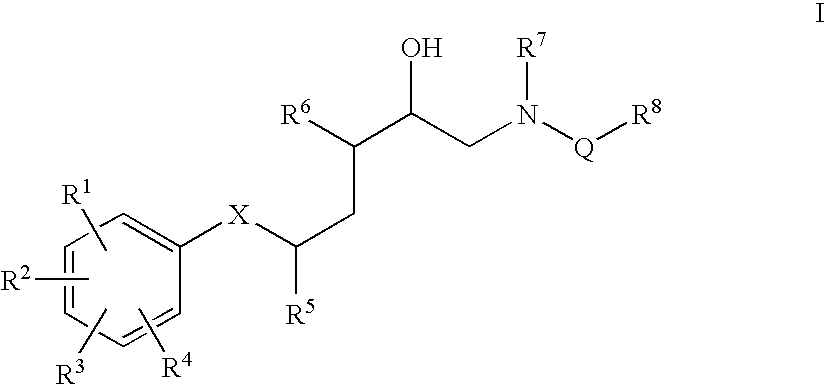
Diaminopropanol Renin Inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Amide compounds and use of the same
InactiveUS20100324010A1Superior renin inhibitory activityHigh activityBiocideOrganic chemistryOrgan damageMedicinal chemistry
A renin inhibitor comprising a compound represented by the formula:wherein each symbol is as defined in the description, or a salt thereof or a prodrug thereof. The compound of the present invention has a superior renin inhibitory activity, and thus is useful as an agent for the prophylaxis or treatment of hypertension, various organ damages attributable to hypertension and the like.
Owner:TAKEDA PHARMA CO LTD
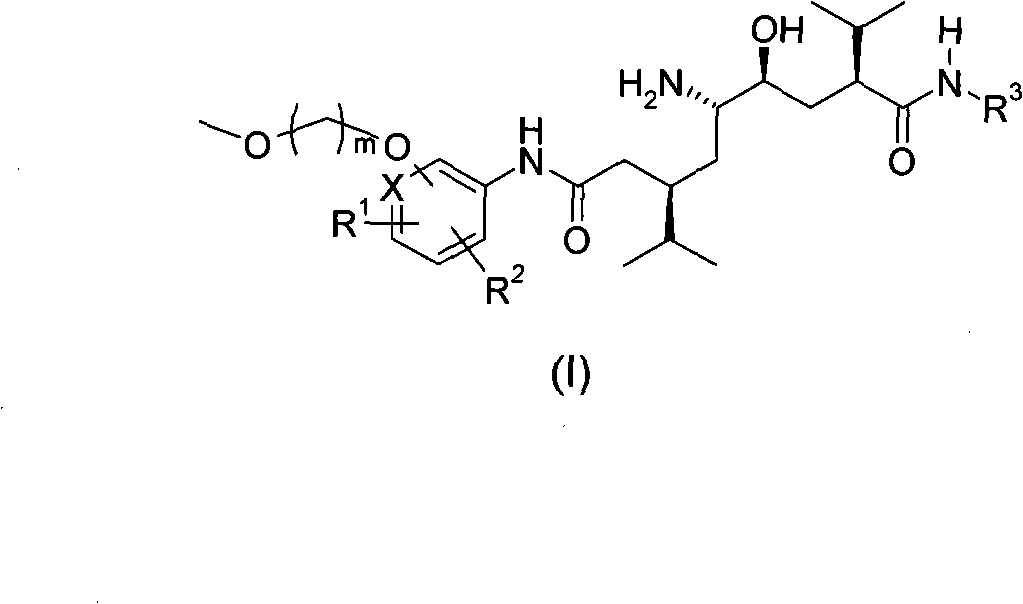
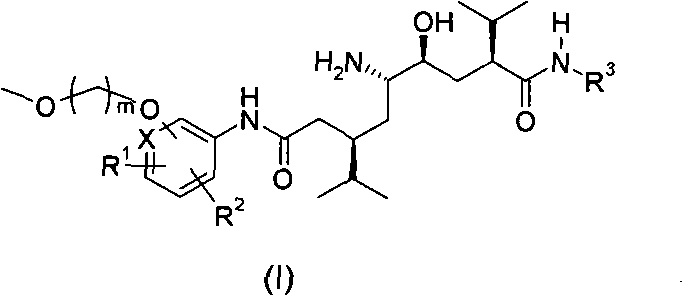
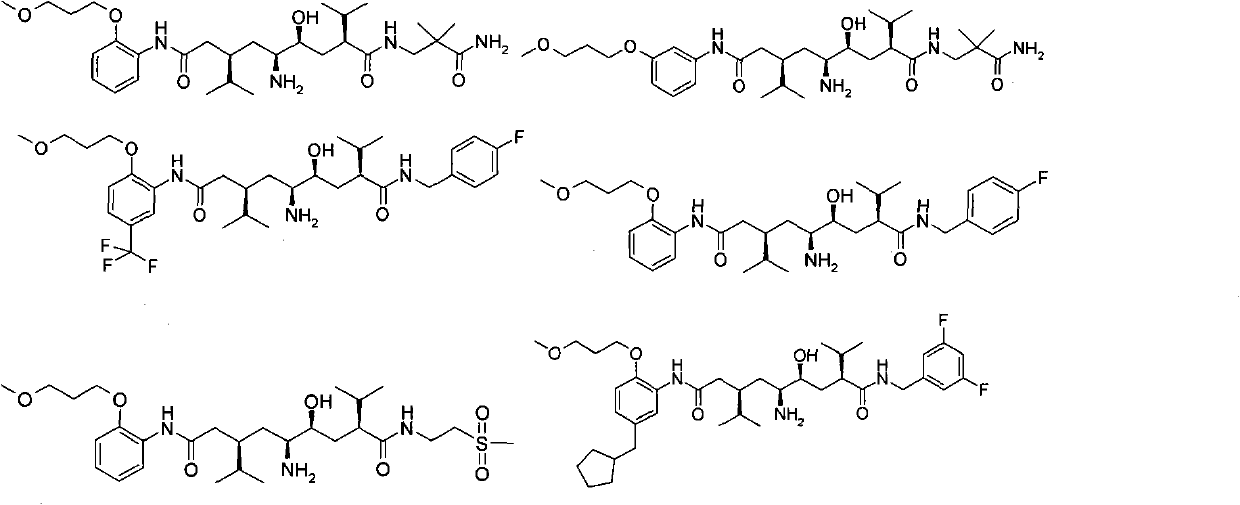
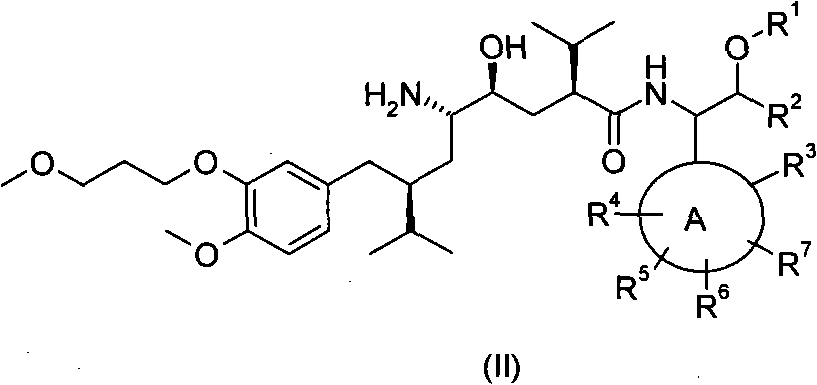
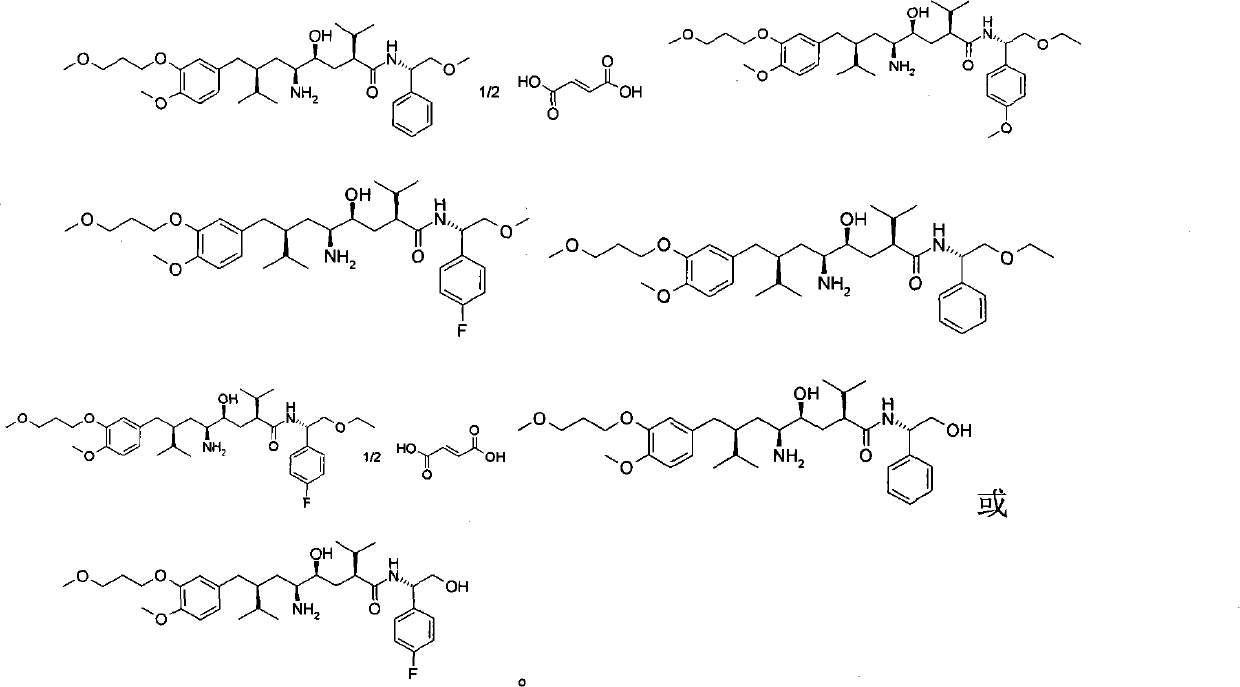
5-amino-4-hydroxy-N-aryl azelamide derivatives as well as preparation methods and medical applications thereof
InactiveCN102060732APharmacokinetic absorption is goodObvious pharmacokinetic advantageGroup 4/14 element organic compoundsSenses disorderArylResistant hypertension
The invention relates to 5-amino-4-hydroxy-N-aryl azelamide derivatives as well as preparation methods and medical applications thereof, and in particular relates to new 5-amino-4-hydroxy-N-aryl azelamide derivatives shown in the general formula (I) and preparation methods thereof as well as drug compositions containing the derivatives and applications of the derivatives as treatment agents, especially renin inhibitors, and as drugs for treating such diseases related to activity of renin as resistant hypertension, wherein each substituent group in the general formula (I) is as defined in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Renin inhibitors
InactiveUS20100160424A1Low costHigh activityBiocideCarbamic acid derivatives preparationDiseaseAspartic protease activity
Described are compounds that bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also described are methods of use of the compounds described herein in ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
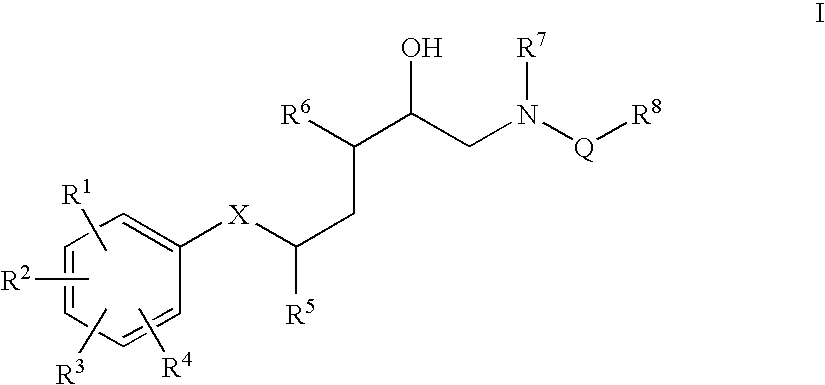
Diaminopropanol renin inhibitors
Described are diaminopropanols of which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with elevated. levels of renin activity or in the treatment of aspartic protease mediated disorders. Also described is a method for the use of the diaminopropanols in ameliorating or treating renin related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
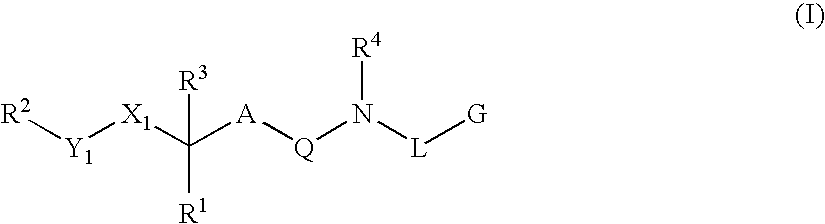
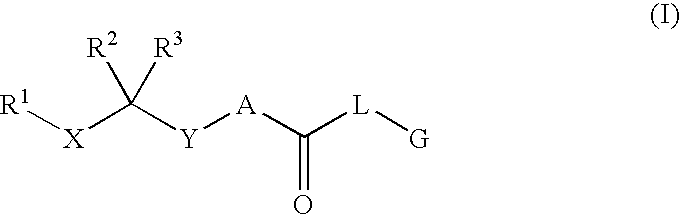
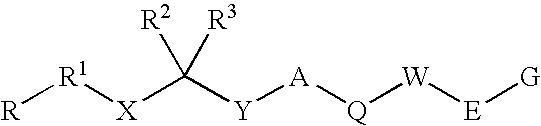
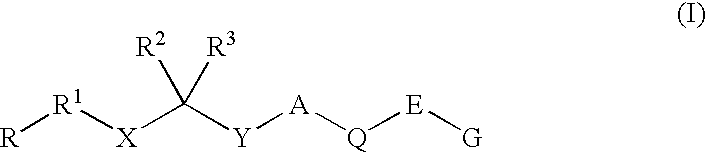
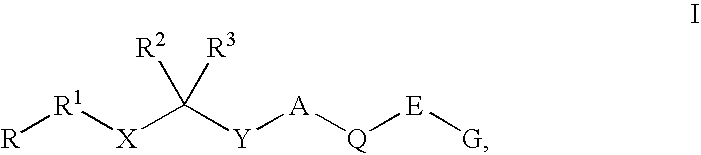
Renin Inhibitors
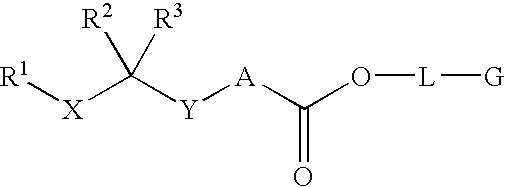
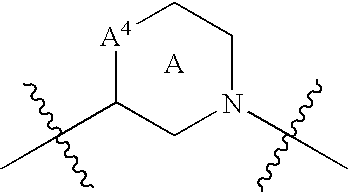
Disclosed are compounds having the formula (I): wherein the R1, R2, R3, X, Y, A, L, and G are defined herein. These compounds bind to aspartic proteases to inhibit their activity and are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also disclosed are methods of use of the compounds of Formula I for ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
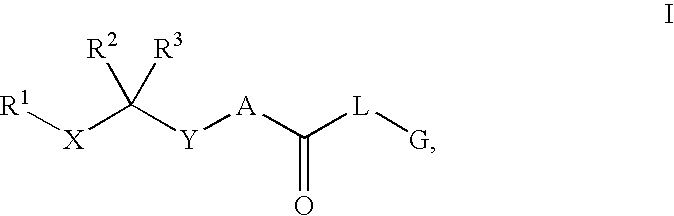
Renin Inhibitors
The present invention is directed to aspartic protease inhibitors represented by the following structural formula (I), or a pharmaceutically acceptable salt thereof. The present invention is also directed to pharmaceutical compositions comprising the aspartic protease inhibitors of Structural Formula (I). Methods of antagonizing one or more aspartic proteases in a subject in need thereof, and methods for treating an aspartic protease mediated disorder in a subject using these aspartic protease inhibitors are also disclosed.
Owner:VITAE PHARMA INC
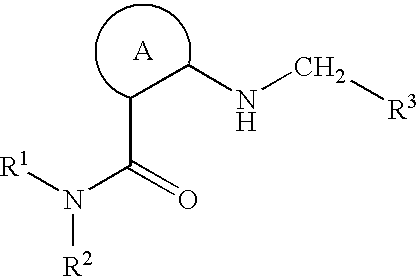
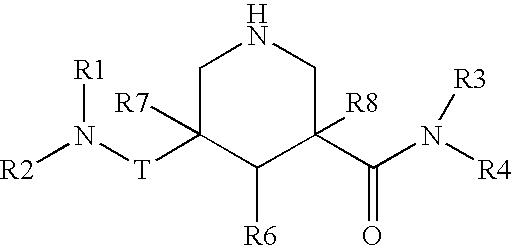
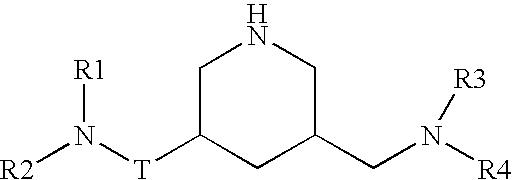
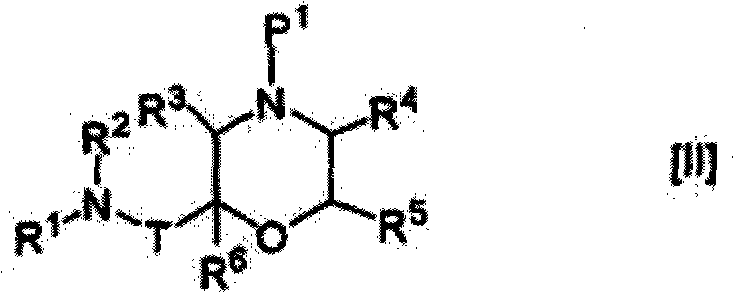
Nitrogen-containing saturated heterocyclic compound
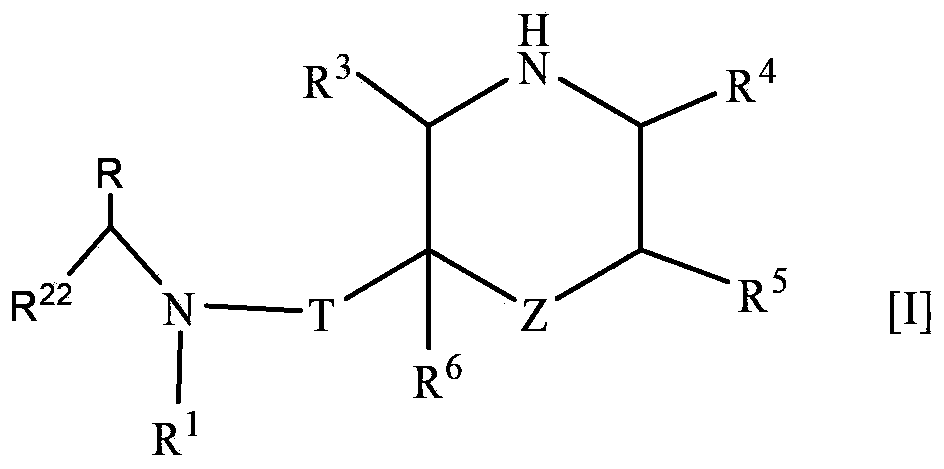
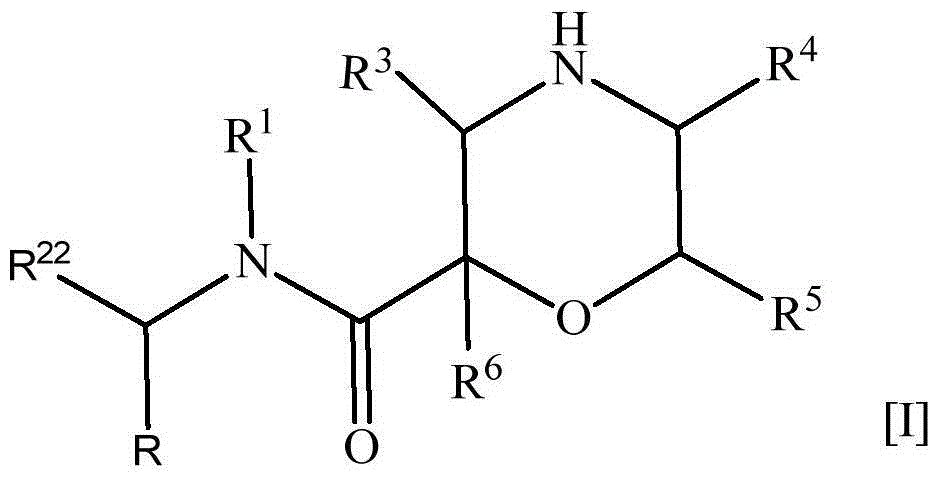
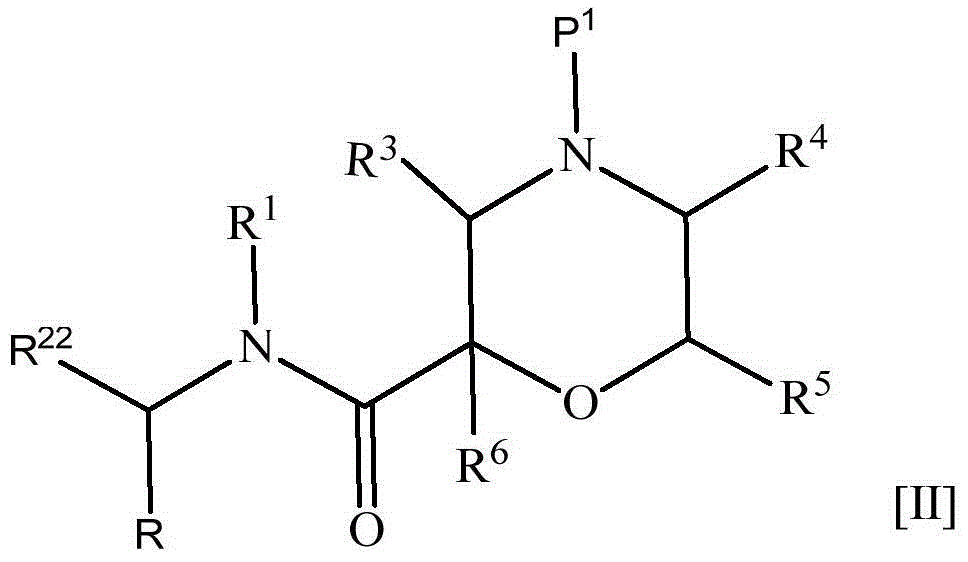
The present invention provides a nitrogen-containing saturated heterocyclic compound of the formula [I]: wherein R 1 is a cycloalkyl group and the like, R 22 is an optionally substituted aryl and the like, R is a lower alkyl and the like, T is a carbonyl group, Z is -O- and the like, and R 3 to R 6 are the same or different and a hydrogen atom and the like; or a pharmaceutically acceptable salt, that is useful as a renin inhibitor.
Owner:SHANGAI PHARMA GRP CO LTD +1
Cardiovascular Compounds Comprising Nitric Oxide Enhancing Groups, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one nitric oxide enhancing group, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; Q) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The cardiovascular compounds are angiotensin II antagonists, aldosterone antagonists, endothelin antagonists, hydralazine compounds, neutral endopeptidase inhibitors and renin inhibitors. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors.
Owner:NICOX SA
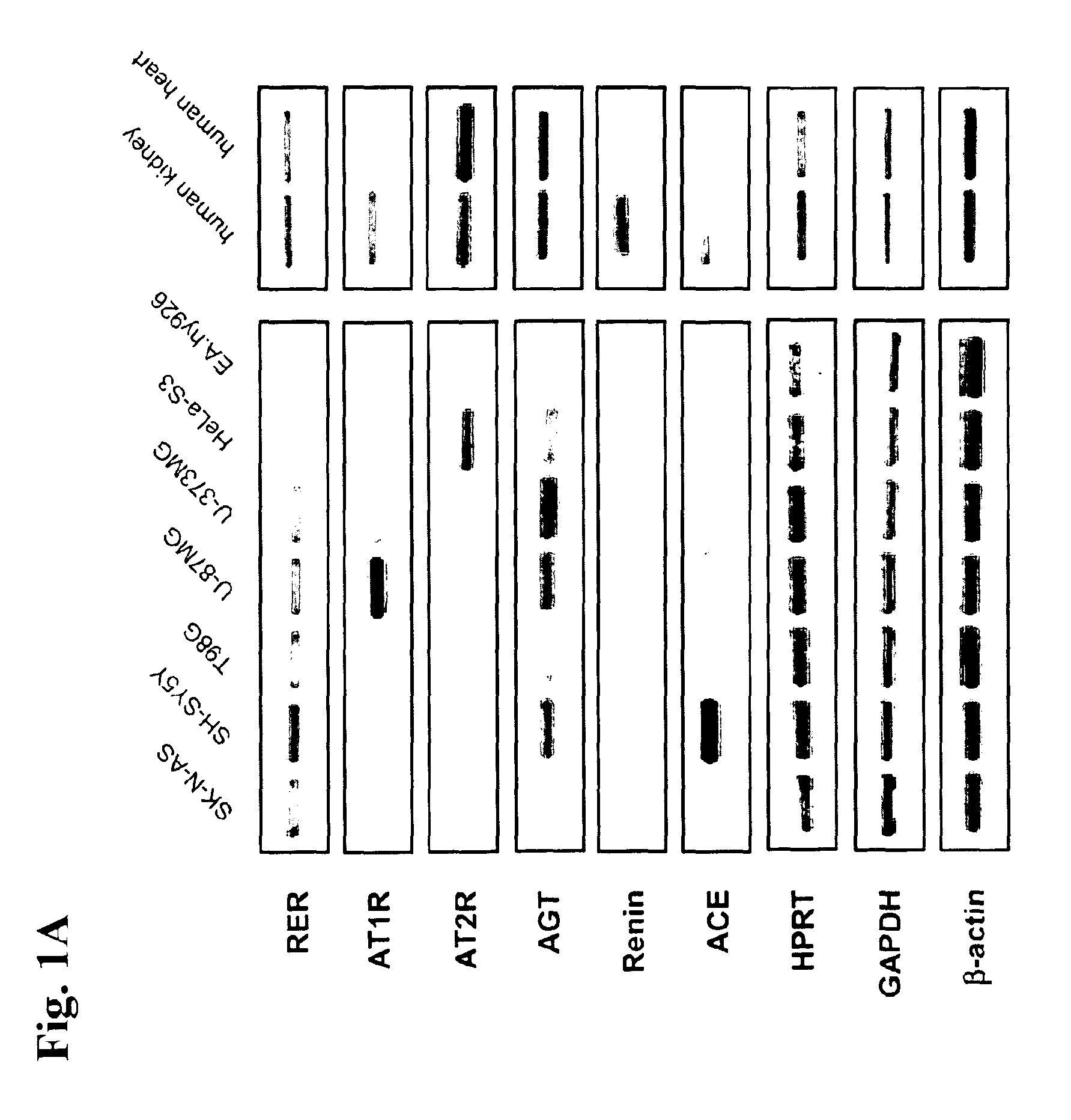
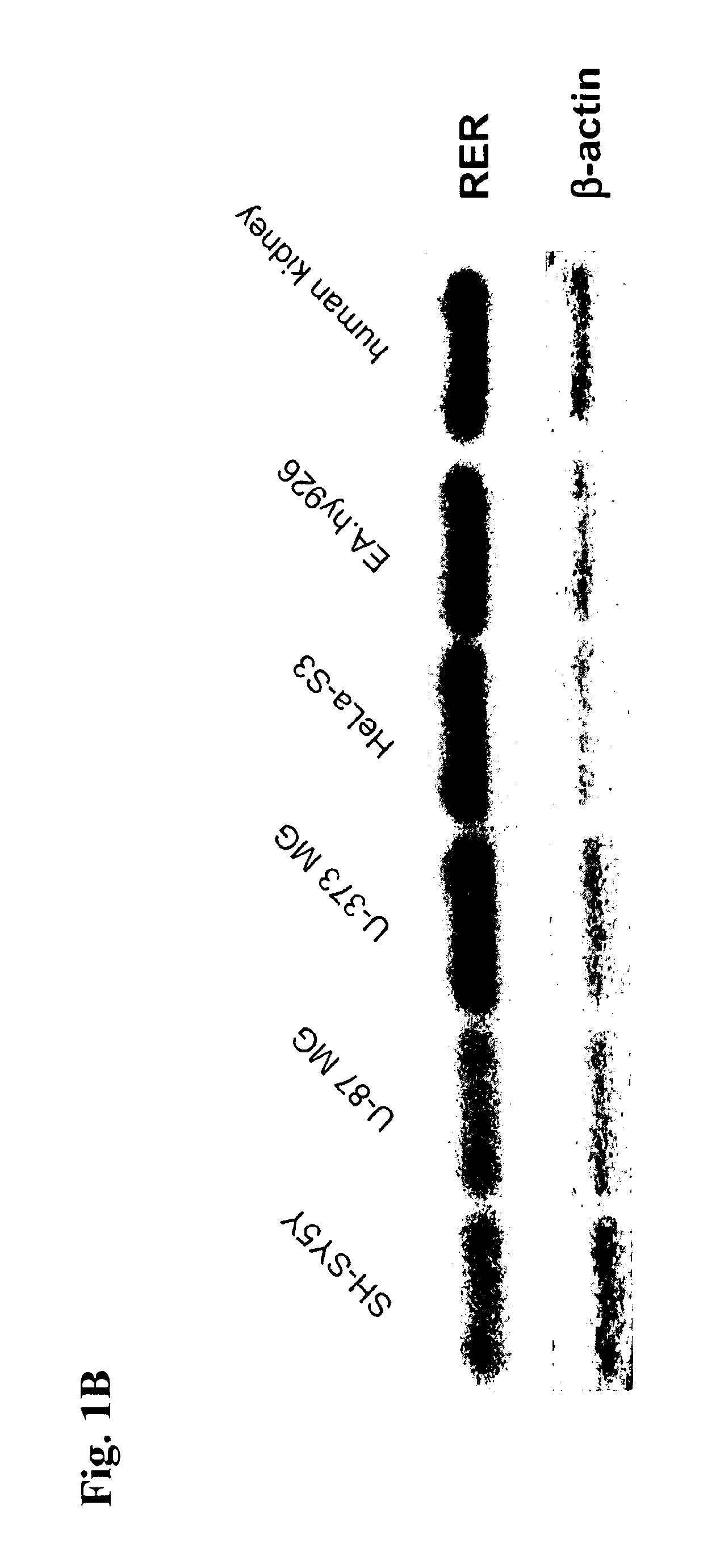
Determination of renin-prorenin receptor activity
InactiveUS20110190142A1Less susceptibleSugar derivativesPeptide/protein ingredientsSide effectPharmaceutical medicine
The present invention refers to the renin / prorenin receptor (RER) signal transduction pathway and, in particular, to the role of promyelocytic zinc finger protein (PLZF) and its downstream targets involved in this pathway, e.g. the p85α subunit of phosphatidylinositol-3 kinase (PI3K-p85α). In more detail, the present invention refers to a method for determination of RER activity, e.g. stimulation or inhibition of RER activity, using PLZF activity as a measurement from which RER activity is derived. For the determination of RER activity, use can be made of RER / PLZF protein interaction, PLZF translocation and / or PLZF recruitment. The present invention further refers to a use of said method for identifying RER ligands, e.g. pharmaceutically active agonists or antagonists, as well as for studying undesired side-effects of renin inhibitors.
Owner:CHARITE UNIVS MEDIZIN BERLIN
5-amino-4-hydroxy-7-benzyl-8-methylnonanamide derivative, preparation method thereof and application thereof in medicines
InactiveCN102120724APharmacokinetic absorption is goodObvious pharmacokinetic advantageOrganic active ingredientsSenses disorderDiseaseMethyl group
The invention relates to a 5-amino-4-hydroxy-7-benzyl-8-methylnonanamide derivative, a preparation method thereof and application thereof in medicines. Specifically, the invention relates to a novel 5-amino-4-hydroxy-7-benzyl-8-methylnonanamide derivative shown in a general formula (I) in the specification, a preparation method thereof, and medicinal composition containing the derivative as well as application of the medicinal composition as a therapeutical agent, particularly as a Renin inhibitor and medicaments for treating and resisting high blood pressure and diseases related to Renin activities, wherein all substituent groups in the general formula (1) are identical to those defined in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
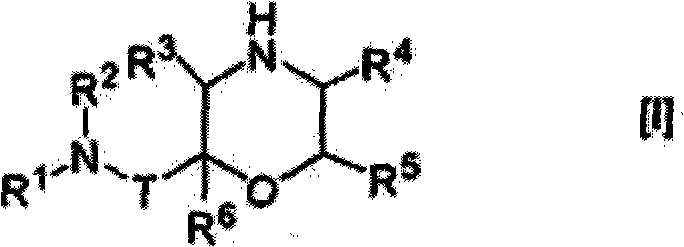
Morpholine derivative
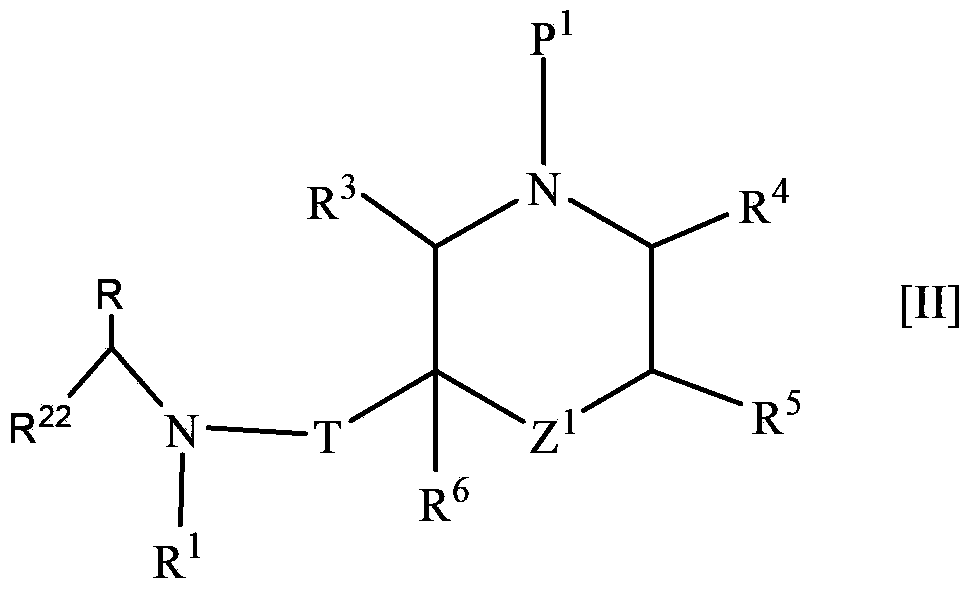
The present invention provides a morpholine derivative of the formula ¢I!; wherein R 1 is a substituted alkyl group, an optionally substituted aryl group, an optionally substituted heterocyclo group,a cycloalkyl group or an alkyl group; R 2 is a substituted alkyl group, an optionally substituted aryl group, an optionally substituted heterocyclo group, an optionally substituted alkylcarbonyl group, an optionally substituted arylcarbonyl group, an optionally substituted heterocyclo-substituted carbonyl group or a cycloalkylcarbonyl group; T is a methylene group or a carbonyl group; R 3 , R 4 ,R 5 and R 6 are the same or different and a hydrogen atom, an optionally substituted carbamoyl group or an optionally substituted alkyl group; or pharmaceutically acceptable salts thereof. These compounds are useful as a renin inhibitor.
Owner:MITSUBISHI TANABE PHARMA CORP +1
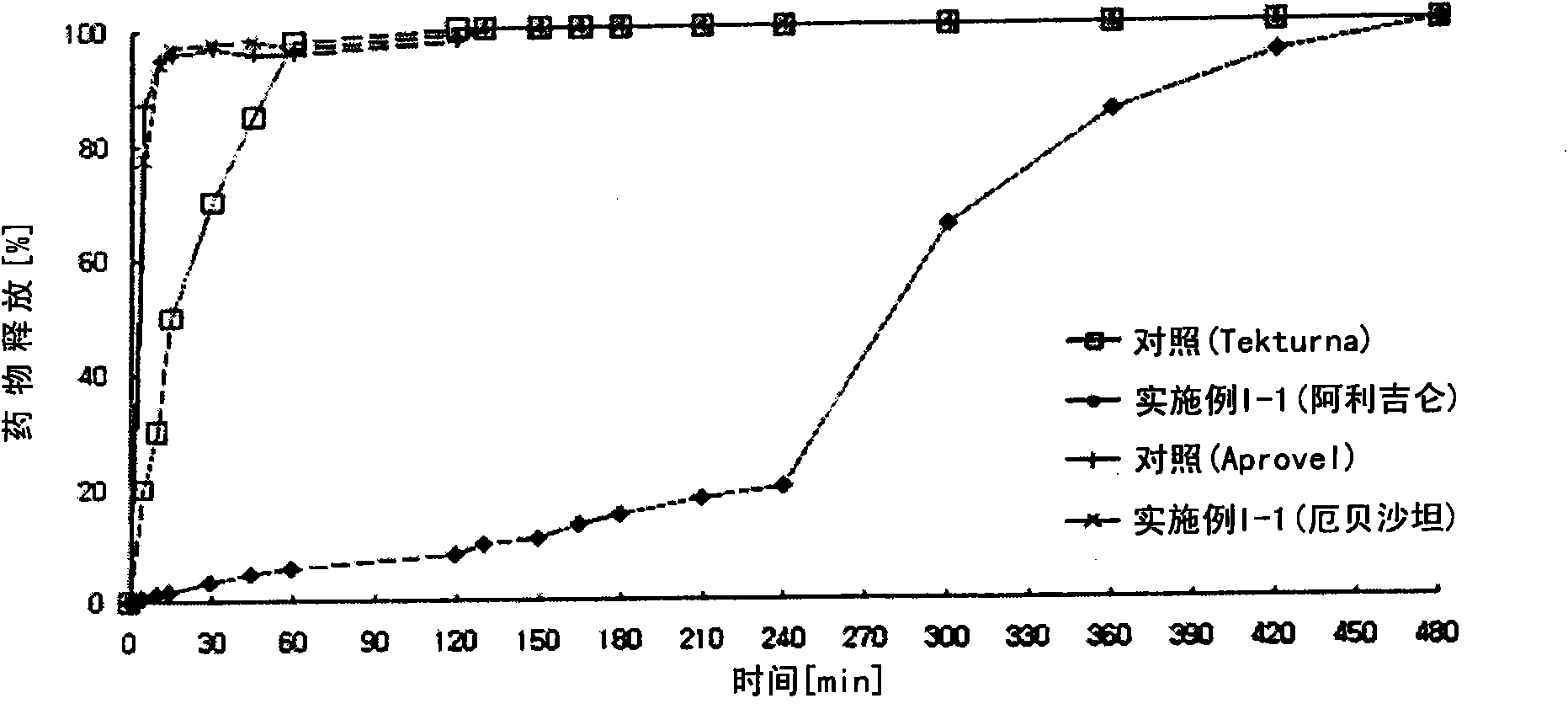
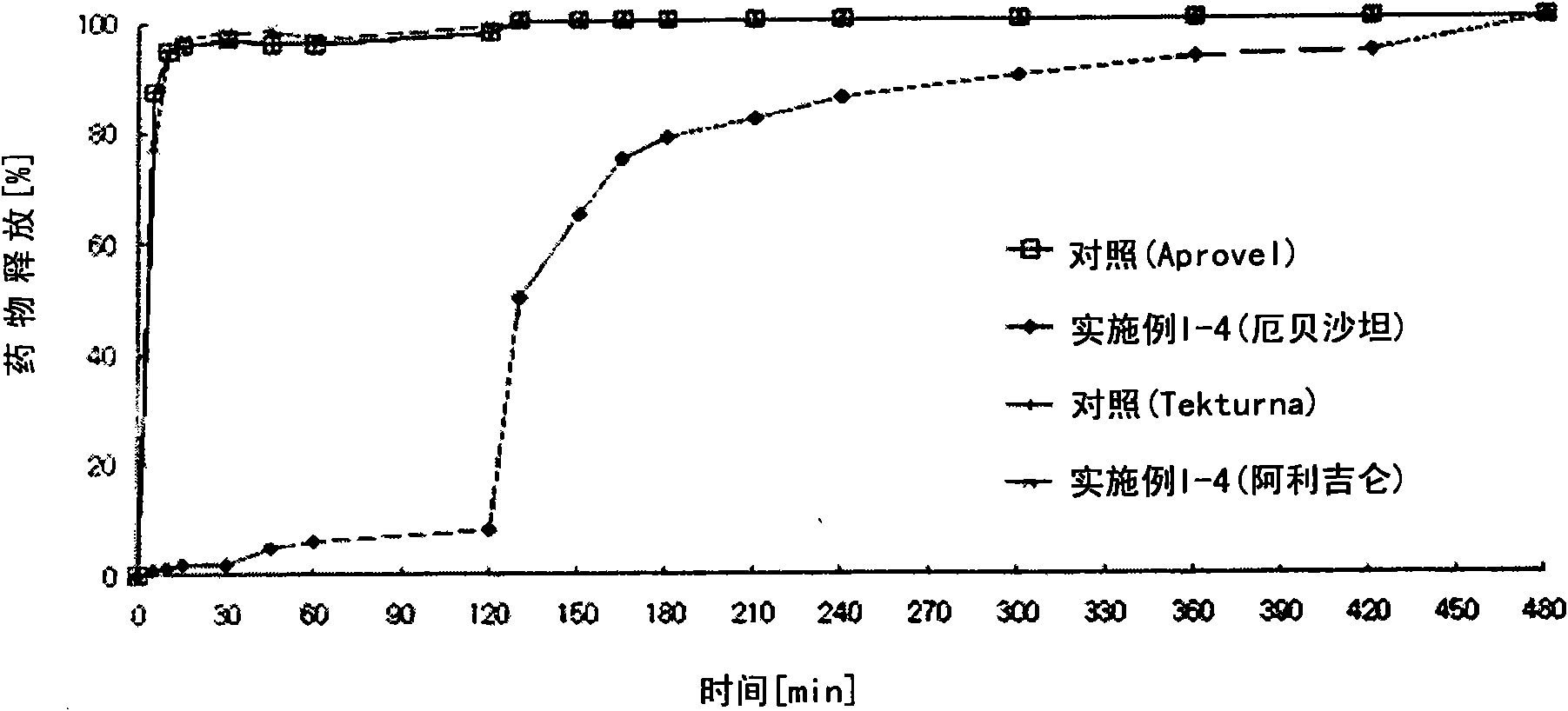
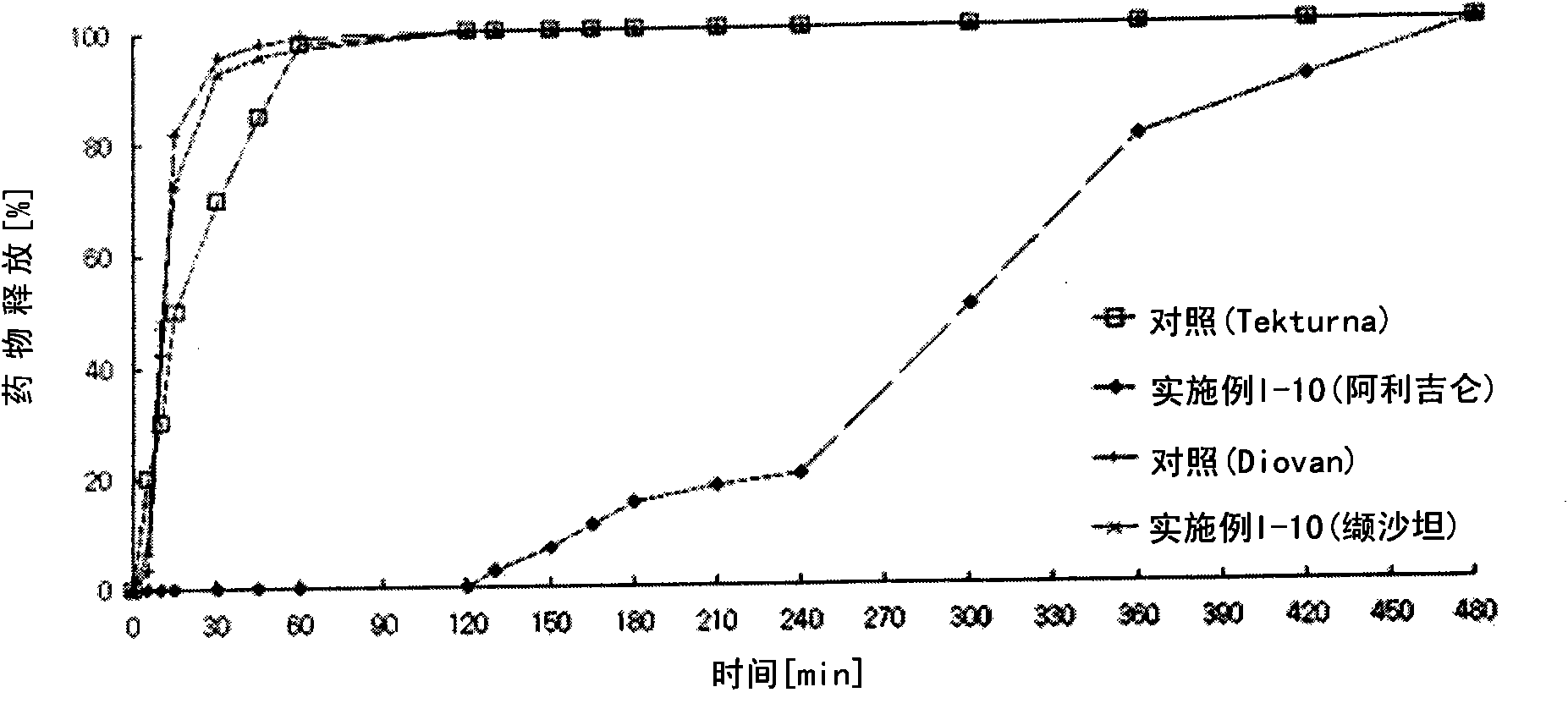
Pharmaceutical formulation
InactiveCN101990428AImprove complianceLittle side effectsMetabolism disorderGranular deliverySide effectAdditive ingredient
The present invention provides a pharmaceutical formulation comprising a compartment containing a rennin inhibitor as a pharmacologically active ingredient, and a compartment having an angiotensin-II-receptor blocker as a pharmacologically active ingredient. One of the compartments is an immediate-release compartment and the other one is an extended-release compartment. Since the disclosed formulation delivers the rennin inhibitor and angiotensin-II-receptor blocker at a specific delivery rate at a different time. It has an advantage in reducing the concern about side effects, improving drug effects, and simplifying the instructions for use of the drug. In addition, the formulation can pharmacologically, clinically, scientifically, and economically achieve more useful effects than the complex prescription case of taking the ingredients separately or each at once, in preventing and treating metabolic syndrome, cardiovascular disease and renal disease.
Owner:HANALL PHARMA CO LTD
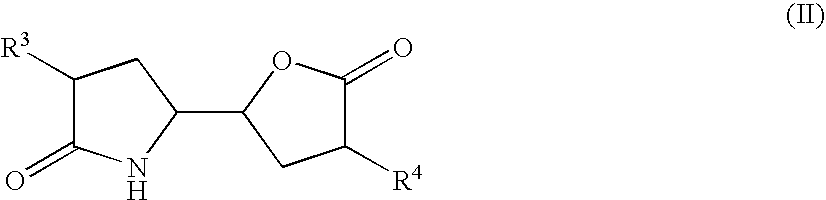
3-alkyl-5- (4-alkyl-5-oxo-tetrahydrofutran-2-yl) pyrrolidin-2-one derivatives as intermediates in the synthesis of renin inhibitors
ActiveUS7772405B2Simple manufacturing methodHigh purityCarbamic acid derivatives preparationOrganic compound preparationSimple Organic CompoundsKetone
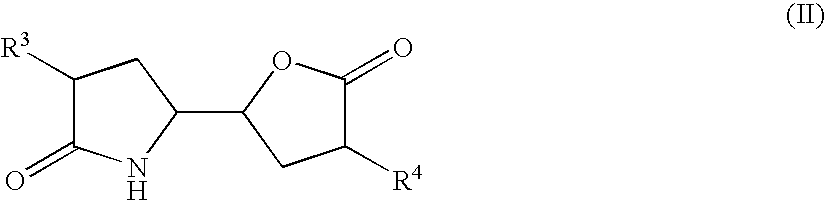
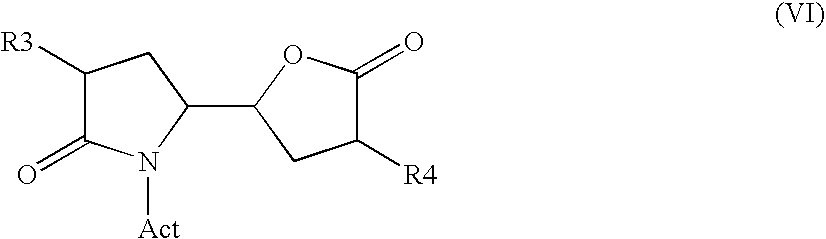
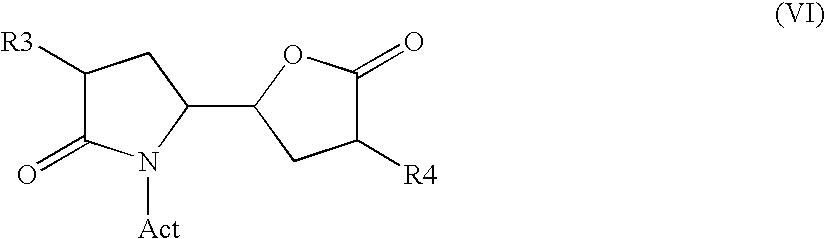
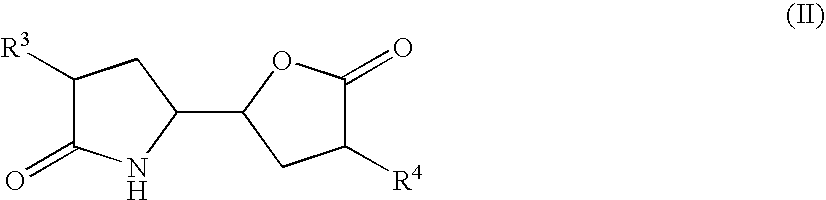
The invention related to a novel process, novel process steps and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially renin inhibitors, such as Aliskiren. Inter alia, the invention relates to a process for the manufacture of a compound of the formula II,or a salt thereof, and a compound of formula VIor a salt thereof, wherein R3 and R4 as well as Act are as defined in the specification, and processes of manufacturing these.Additionally transformation of compounds (VI) with metallo organic compounds (VII) give rise to the new compounds (VIII) which are direct precursors for the preparation of Aliskiren.
Owner:NODEN PHARMA DAC
Piperidine and morpholine renin inhibitors
Described are compounds which are orally active and bind to renin to inhibit its activity. They are useful in the treatment or amelioration of diseases associated with renin activity. Also described are methods of use of these compounds for treating or ameliorating a renin mediated disorder in a subject.
Owner:VITAE PHARMA INC
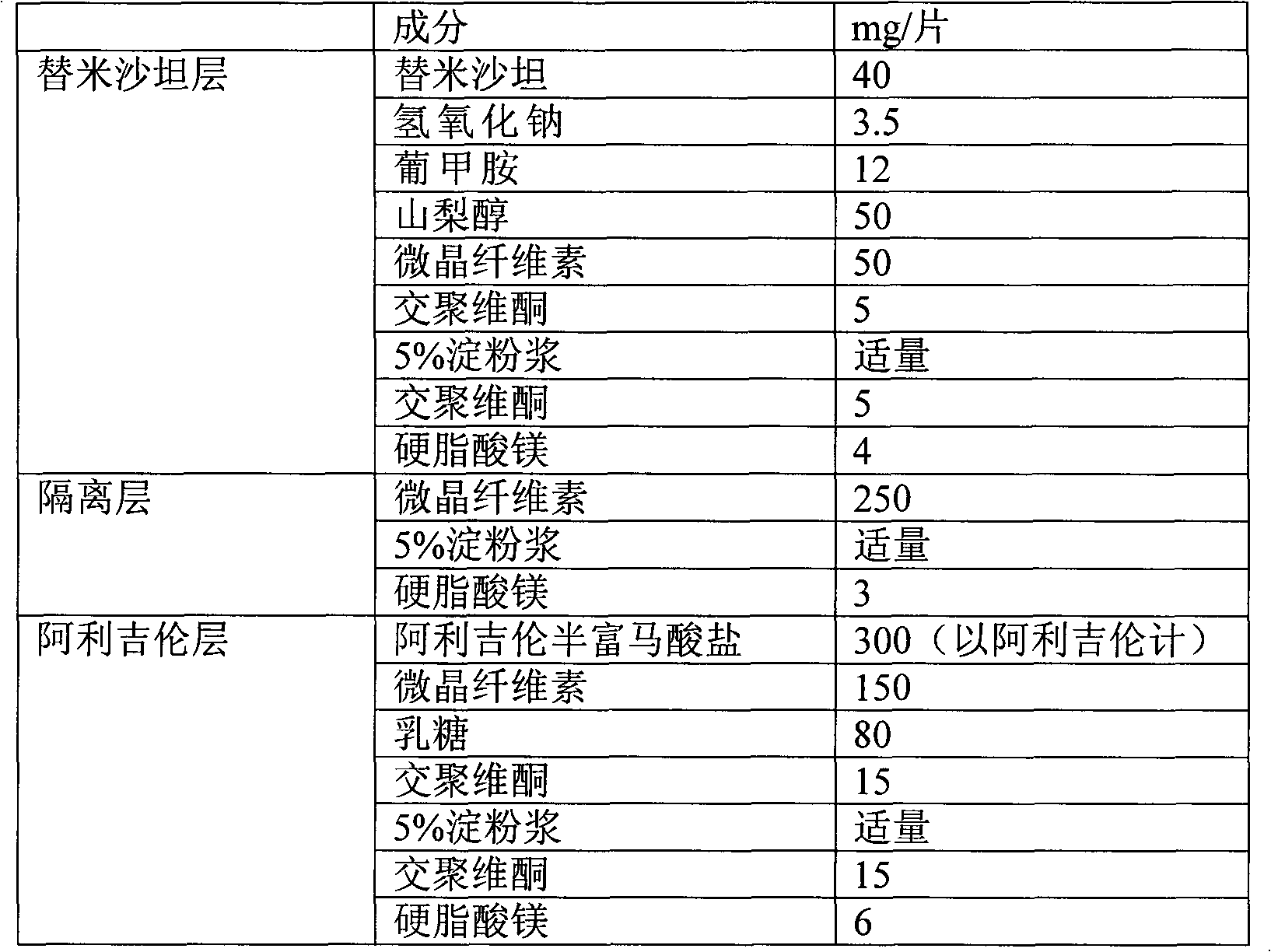
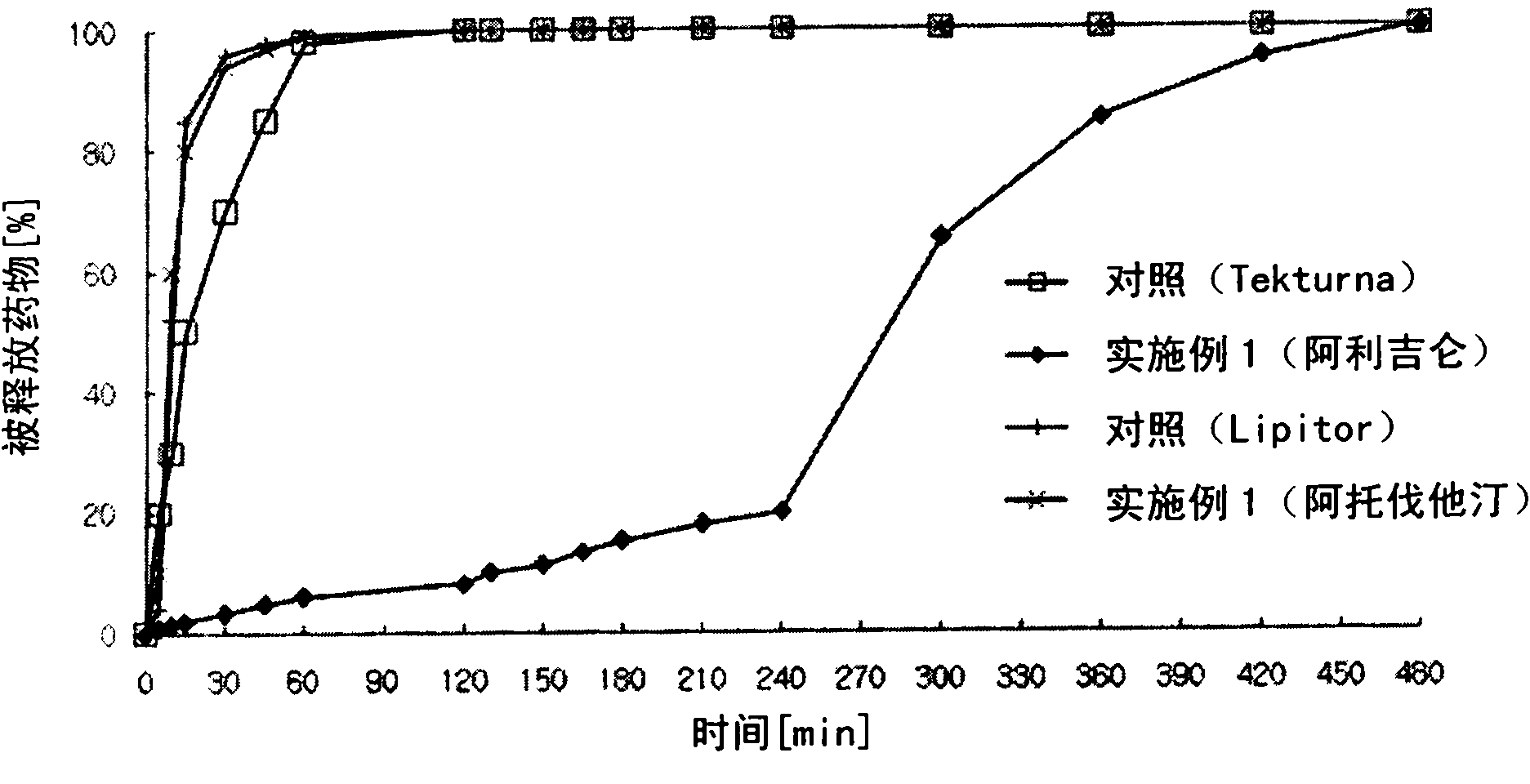
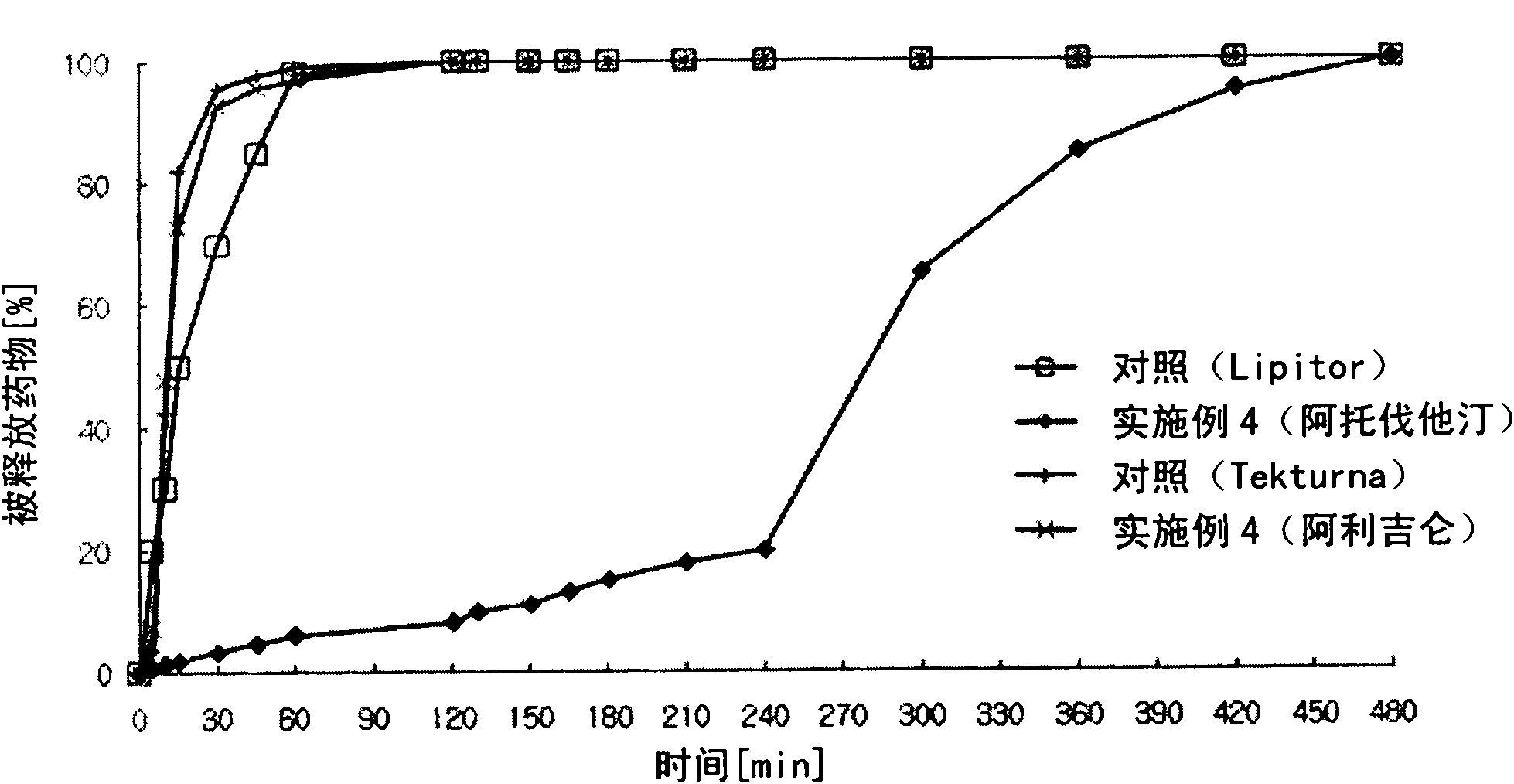
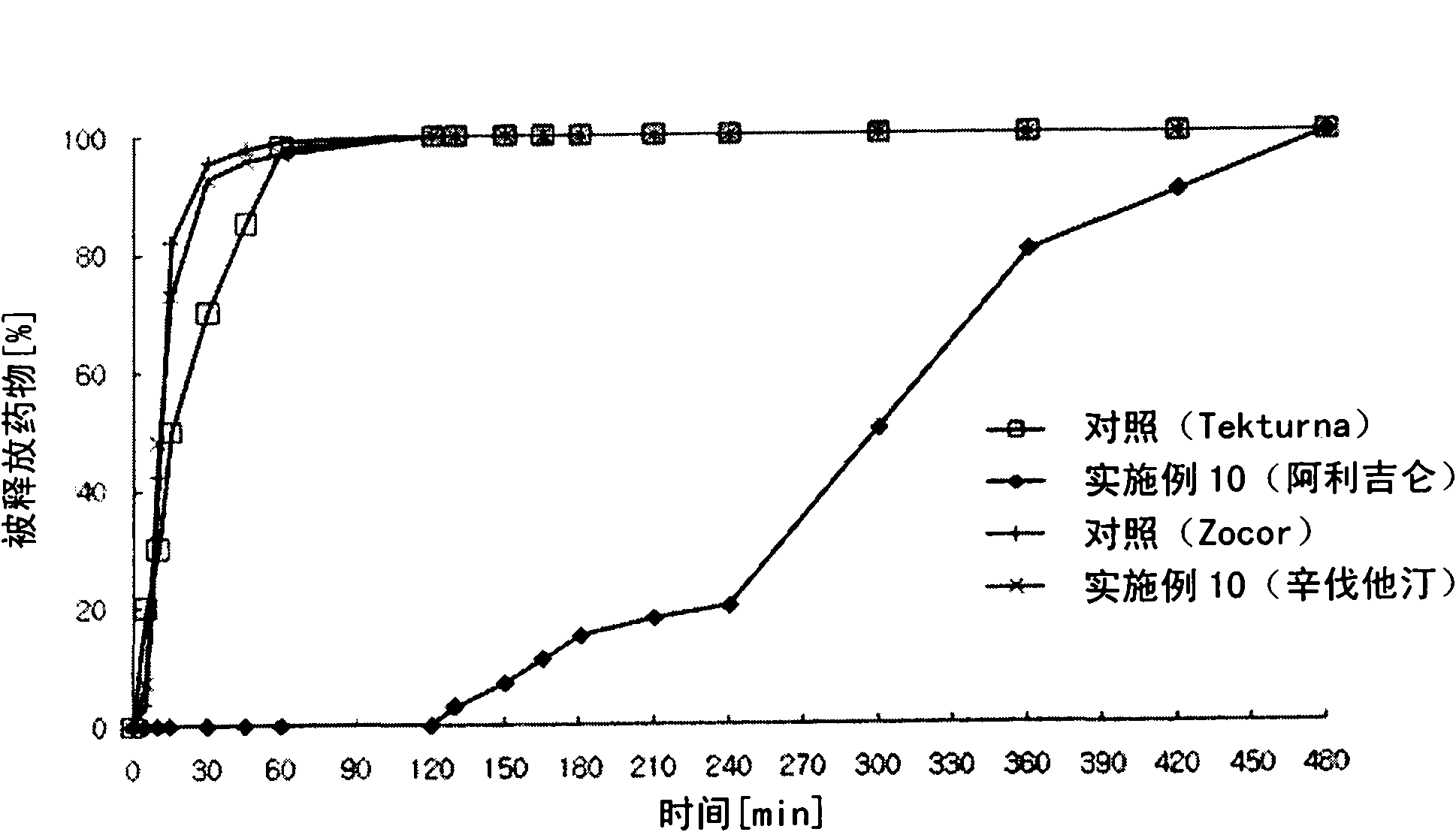
Combined medicament containing telmisartan and aliskiren and preparation method thereof
ActiveCN101926793AAvoid interactionGuaranteed stabilityOrganic active ingredientsPill deliveryPharmaceutical formulationTreatment hypertension
The invention relates to application of telmisartan and aliskiren or medicinal salts thereof in preparing a combined medicament for treating hypertension. The invention also provides the combined medicament for treating hypertension, wherein the combined medicament contains unit preparations of different specifications, and the preparations administrated simultaneously, respectively or in turn are prepared from telmisartan and aliskiren or medicinal salts thereof and pharmaceutically acceptable carriers. The invention also provides a preparation method and application of the combined medicament. The medicinal preparations are three-layer tablets so as to avoid interaction of the aliskiren and the telmisartan, improve the medicament stability and facilitate long-term storage. The composition preparations are used for treating medium and high hypertension patients and hypertension patients whose blood pressure cannot be fully controlled after being treated by angiotensin II receptor antagonists or renin inhibitors.
Owner:CHENGDU ZIHAO PHARMA
Pharmaceutical preparation
InactiveCN102014881AImprove complianceLittle side effectsMetabolism disorderPill deliveryDosing regimenSide effect
Disclosed is a pharmaceutical preparation including a compartment containing a renin inhibitor as a pharmacologically active component, and a compartment containing HMG-CoA reductase inhibitor as a pharmacologically active component, wherein one of compartments is an advance release compartment and the other of the compartments is a retard release compartment. The combination preparation of the present invention delivers the renin inhibitor and HMG-CoA reductase inhibitor with a time difference at a specific speed, reducing undesirable side-effects, improving the potentiating effect and allowing for ease in teaching dosing regimens and enhanced patient compliance. Further, the pharmaceutical preparation of the present invention has pharmacological, clinical, scientific and economical advantages in the prevention or treatment of metabolic syndrome, cardiovascular disease, renal disease and the like as compared with the complex drug regimens in which medicament ingredients are taken individually or simultaneously.
Owner:HANALL PHARMA CO LTD
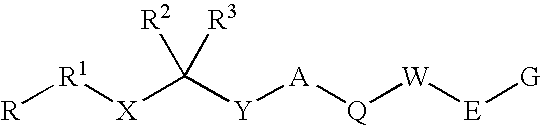
1-Acylamino-2-Hydroxy-3-Amino- -Arylalkanes as Renin Inhibitors
Owner:VITAE PHARMA INC
Use of cicletanine and other furopyridines for treatment of systolic-predominant hypertension, isolated systolic hypertension, elevated pulse pressure, and general hypertension
InactiveUS20070105817A1EffectivenessImprove bioavailabilityBiocideAnimal repellantsHypertension medicationsBeta blocker
This invention provides therapeutic compositions of cicletanine and other furopyridines for the treatment of, elevated pulse pressure or isolated systolic hypertension, as well as general hypertension, in monotherapy and in combined therapy with other anti-hypertensive agents (such as organic and inorganic nitrogen donors, calcium channel blockers, diuretics, beta blockers, angiotensin receptor blockers, ACE inhibitors, aldosterone antagonists, renin inhibitors and centrally-acting antihypertensives) cardiovascular agents (such as medications to treat heart failure) and oral antidiabetic agents (such as biguanides and glitazones). Such compositions include enantiomerically pure (positive or negative) embodiments, as well as enantiomeric mixtures other than a racemic mixture, and include daily dosages of less than 50 mg. Further provided are methods of treatment of general or systolic hypertension, wherein patients are administered the inventive compositions, either a monotherapeutic furopyridine composition, or a combination therapy, which includes a second agent in addition to the furopyridine, for treatment of general hypertension or systolic hypertension, and hypertension-associated complications.
Owner:GILEAD SCI INC
Renin inhibitors
InactiveUS20090275581A1Low costHigh activityBiocideOrganic chemistryDiseaseAspartic protease activity
Disclosed are compounds according to Formula I:wherein the variables are defined herein. Such compounds are can bind aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity.Also described herein are methods of antagonizing aspartic protease inhibitors in a subject in need thereof comprising administering to the subject a therapeutically effective amount of a compound according to Formula I.
Owner:VITAE PHARMA INC
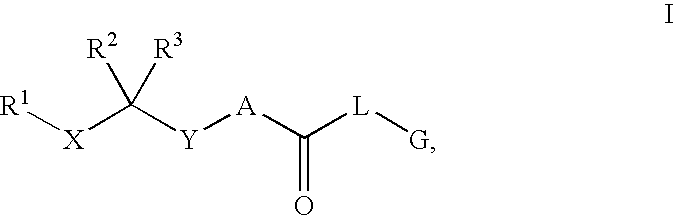
Renin Inhibitors
Described are compounds of the formula (I) which are orally active and bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also described are methods of use of the compounds described herein in ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Renin Inhibitors
Described are compounds which bind to aspartic proteases to inhibit their activity. They are useful in the treatment or amelioration of diseases associated with aspartic protease activity. Also described are methods of use of the compounds described herein in ameliorating or treating aspartic protease related disorders in a subject in need thereof.
Owner:VITAE PHARMA INC
Novel renin inhibitor
ActiveCN104640847AExcellent renin inhibitionOrganic active ingredientsOrganic chemistryArylHydrogen atom
The invention provides a nitrogen-containing saturated heterocyclic compound useful as a renin inhibitor. A compound represented by formula [I] (in the formula, R1 represents a cycloalkyl or alkyl, R22 represents an optionally substituted aryl or the like, R represents a lower alkyl group, R3, R4, R5, and R6 are the same or different and represent a hydrogen atom, optionally substituted carbamoyl, optionally substituted alkyl, or alkoxycarbonyl) or a pharmaceutically acceptable salt thereof.
Owner:SHANGAI PHARMA GRP CO LTD +1
3-Alkyl-5- (4-Alkyl-5-Oxo-Tetrahydrofutr An -2-Yl) Pyrrolidin-2-One Derivatives As Intermediates In The Synthesis Of Renin Inhibitors
ActiveUS20080262246A1High diastereomeric purityHigh enantiomeric purityCarbamic acid derivatives preparationLithium organic compoundsKetoneOrganic compound
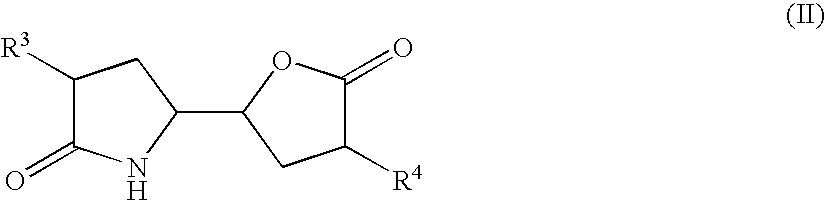
The invention related to a novel process, novel process steps and novel intermediates useful in the synthesis of pharmaceutically active compounds, especially renin inhibitors, such as Aliskiren. Inter alia, the invention relates to a process for the manufacture of a compound of the formula II,or a salt thereof, and a compound of formula VIor a salt thereof, wherein R3 and R4 as well as Act are as defined in the specification, and processes of manufacturing these.Additionally transformation of compounds (VI) with metallo organic compounds (VII) give rise to the new compounds (VIII) which are direct precursors for the preparation of Aliskiren.
Owner:NODEN PHARMA DAC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com